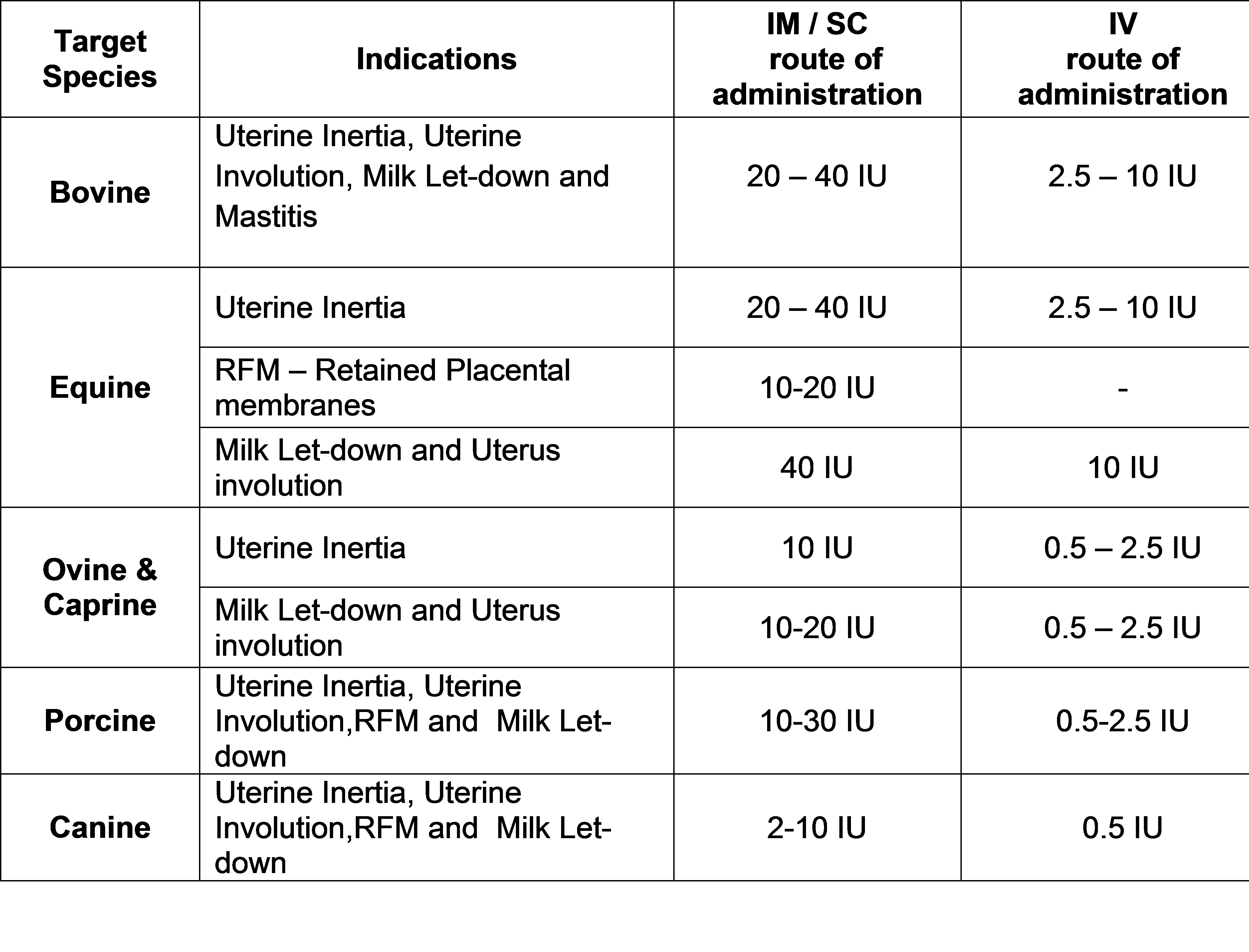Therapeutic Group : ANTIBACTERIALS
Sector : VETERINARY
Mode of action:
Bactericidal in action, inhibits biosynthesis of the cell wall peptidoglycan which leads to cell wall lysis results in death of bacteria.
Spectrum of activity:
Gram + ve bacteria susceptible to penicillin
Reconstitution and Route of administration:
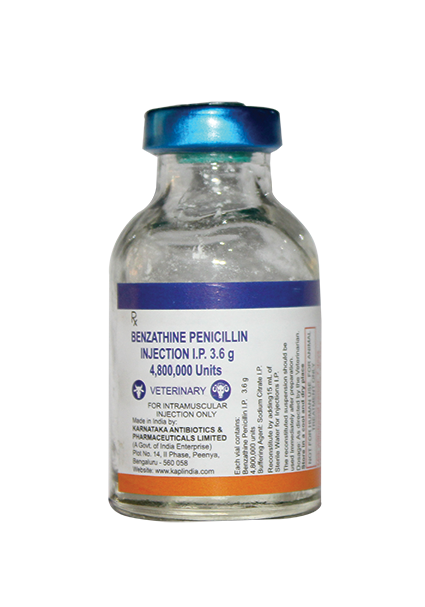
Reconstitute by Adding 15ml sterile water for injection and inject intramuscular immediately.
Withdrawal Period:
Milk: 7 days; Meat: 28 days
Indications:
Actinomycosis, Anthrax, Black leg, Tetanus, Arthritis, Mastitis, Metritis, H.S., Foot rot, Septic wound and GI tract and respiratory tract infections etc.
Contra Indications:
Benzathine Penicillin is not for use in turkeys producing eggs for human consumption or for use in horses intended for food
Dose:
Cow, Buffalo, Sheep, Goat, Pig and Horse: 12,000 IU / kg B.wt
Dog and Cat : 40,000 IU / Kg Body Weight or as directed by a registered veterinary practitioner.