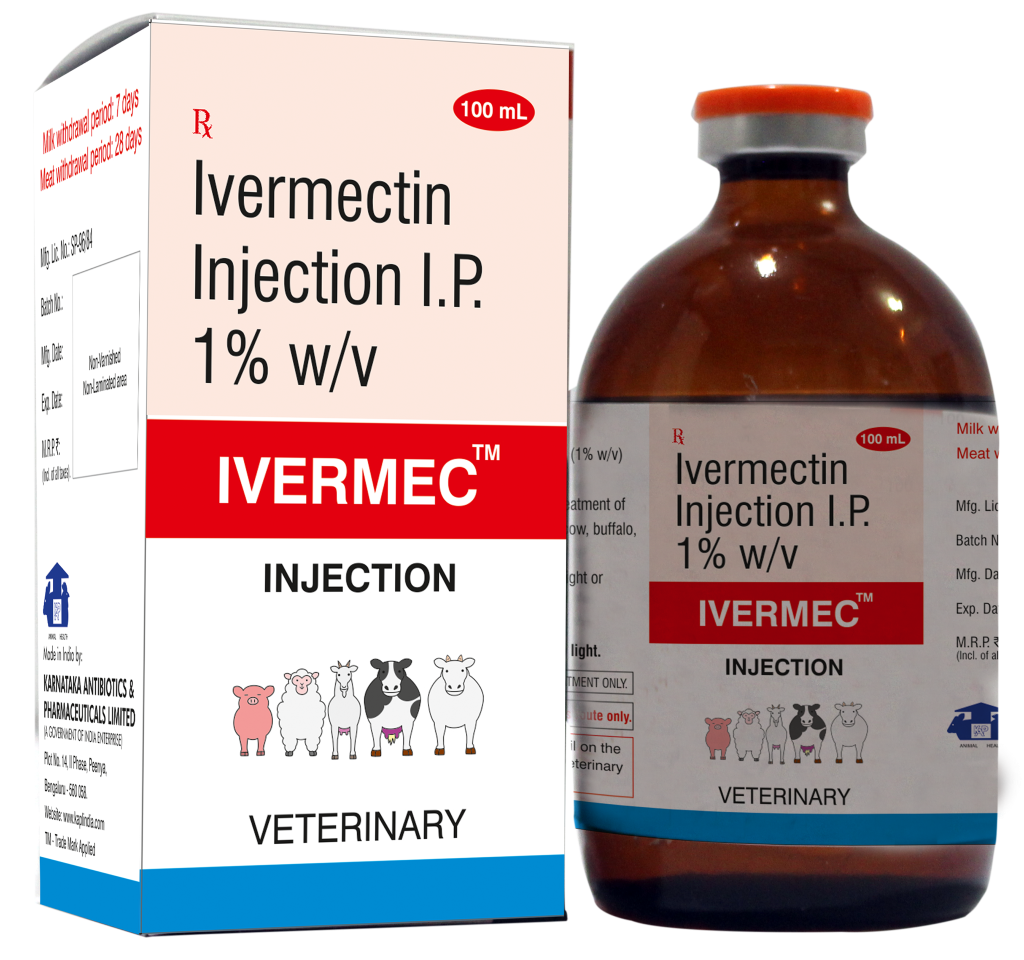
Composition:
Each mL contains:
Ivermectin I.P. 10 mg (1% w/v)
Propylene Glycol q.s.
Indications:
For the control and treatment of endoparasites & ectoparasites in cow, buffalo, sheep, goat & pig.
Dosage:
1 mL per 50 Kg body weight or as directed by the Veterinarian.
Withdrawal Period:
Milk: 7 days
Meat: 28 days
Pack: 100 mL vial
Proven Endectocide

Composition:
Each 100 mL Cal-K gel contains:
Calcium 6600 mg
Phosphorus 3400 mg
Vitamin D3 32000 IU
Vitamin B12 400 mcg
Pueraria mirifica extract 200 mg
Energy Sources
(Propylene Glycol + Glycerol +
Carbohydrate + Aquabase) Q.S.
Indications:
Dose:
For Lactating Dairy Cattle:
To Optimize Milk yield
Administer orally one bottle per animal per day for 5-10 days
To prevent and treat Milk Fever:
Administer one bottle per animal per oral at the time of calving and administer second bottle 6-12 hrs post calving. Repeat every 12 hrs interval as per need. Or as directed by the Veterinarian
Pack: 300 mL HDPE Container
Instant Relief and More Milk
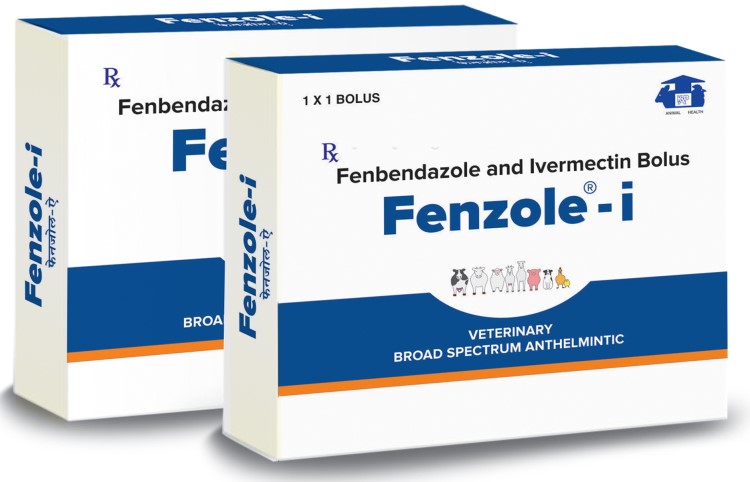
Composition:
Each uncoated bolus contains:
Fenbendazole I.P. 3 gm
Ivermectin I.P. 100 mg
Excipients Q.S.
Indications
To Treat and Prevent Mixed Parasitic Infestation effectively viz. Roundworms, Tapeworms, Flukes & External Parasites(Tick, Lice, Mite & Flea)
Dosage:
Large Animals: 1 bolus per 300 kg bodyweight orally or as directed by the Veterinarian
Withdrawal Period: Milk: 7 days Meat: 28 days
Pack: 3.1 g Bolus with Monocarton
Total Spectrum Anthelmintic
Therapeutic Group : ANTIBACTERIALS
Sector : VETERINARY
Mode of action:
Bactericidal in action, inhibits biosynthesis of the cell wall peptidoglycan which leads to cell wall lysis results in death of bacteria.
Spectrum of activity:
Gram + ve bacteria susceptible to penicillin
Reconstitution and Route of administration:
Reconstitute by Adding 15ml sterile water for injection and inject intramuscular immediately.
Withdrawal Period:
Milk: 7 days; Meat: 28 days
Indications:
Actinomycosis, Anthrax, Black leg, Tetanus, Arthritis, Mastitis, Metritis, H.S., Foot rot, Septic wound and GI tract and respiratory tract infections etc.
Contra Indications:
Benzathine Penicillin is not for use in turkeys producing eggs for human consumption or for use in horses intended for food
Dose:
Cow, Buffalo, Sheep, Goat, Pig and Horse: 12,000 IU / kg B.wt
Dog and Cat : 40,000 IU / Kg Body Weight or as directed by a registered veterinary practitioner.

Composition:
Each 5 mL contains
Tricholine Citrate………………………………………… 750 mg
Inositol……………………………………………………….. 12 mg
Niacinamide ………………………………………………….50 mg
Vitamin B12 ………………………………………………….10 mcg
L- Carnitine………………………………………………….. 10 mg
DL – Methionine…………………………………………….. 8 mg
Ferrous Gluconate………………………………………… 33 mg
Ferrous Chloride…………………………………………… 13 mg
Calcium Lactate……………………………………………. 50 mg
Eclipta Alba………………………………………………….. 35 mg
Phyllanthus niruri………………………………………….. 35 mg
Andrographis Paniculata………………………………… 35 mg
Liver extract with D.M water as……………………….. QS
Indications:
Usage:
Livestock
Small animal: 5 mL / day
Large animal: 25-30 mL / day
Duration : 5-7 days
Poultry for 100 birds
Chicks: Day old – 2 weeks : 10 mL / day
Broiler / Growers / Layers : 15-20 mL / day
Breeders : 50-60 mL / day
or as advised by the Veterinarian
Pack

Therapeutic Group : Anti-infective/ Antiseptics
Sector : Human
Composition:
Isopropyl Alcohol (99.8%) IP : 75% v/v
Hydrogen Peroxide Solution : 0.125% v/v
Glycerol I.P : 1.45% v/v
Purified Water I.P : Q/S
Special Characteristics:
Directions for use:
Apply a palmful of alcohol based hand sanitizer all over surface of the Hand, rub hands until dry.
Availability: Available in 500 ml & 200 ml Packs.

Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each 5ml contains:
Cefixime 50MG
Indication:
Otitis Media
Pharyngitis, Bronchitis, Pneumonia.
Urinary Tract Infection
Enteric Fever
Presentation & Pack:
VIAL
Doctor’s Section:
Medical Information
Therapeutic Group: ANALGESICS
Sector: HUMAN
Composition:
Each tablet contains:
Tramadol HCl 37.5 mg + Paracetamol 325 mg
Indication:
Arthritic pain – ostearthritis, Rheumatoid arthritis, Diabetic neuropathy,Trigeminal neuralgia,Post herpetic neuralgia, Pain due to fractures,Disc prolapse, Burns,Sciatica,Post surgical pain & dental pain, Cancer pain
Presentation & Pack:
10X10’s box
Doctor’s Section:
Medical Information
Therapeutic Group: ANALGESICS (NSAIDs)
Sector: HUMAN
Composition:
Each tablet contains:
Aceclofenac 100 mg
Paracetamol 325 mg
Serratiopeptidase 15 mg
Indication:
Presentation & Pack:
10X10’s box
Doctor’s Section:
Medical Information
Therapeutic Group: PROBIOTIC
Sector: HUMAN
Composition:
Each mini bottle(5 ml) contains:
Bacillus clausii spores 2 billion in 5 ml
Indication:
Presentation & Pack:
5 ml mini bottle
Doctor’s Section:
Medical Information
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each Vial contains:
Merpenem 1GM
Indication:
Bacterial Meningitis &Septicemia
Intra-abdominal Infection
Urinary Tract Infection
Nosocomial Pneumonia
Presentation & Pack:
VIAL
Doctor’s Section:
Medical Information

Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each Vial contains:
Piperacillin 4GM + Tazobactum 0.5GM
Indication:
Septicimea
Nosocomial Bacterial Infection
Surgical Prophylaxis
Presentation & Pack:
VIAL
Doctor’s Section:
Medical Information
Therapeutic Group: ANTIBIOTICS
Sector: HUMAN
Composition:
Each vial contains:
Sterile Ceftriaxone Sodium 1gm
Indication:
Bacterial meningitis, Bacterial septicemia,Surgical prophylaxis,Sexually transmitted diseases,Respiratory tract infections,Urinary tract infections,Serious skin and soft tissue infections,Pelvic inflammatory diseases,Infective endocarditis, Osteomyelitis.
Presentation & Pack:
Individual Vial
Doctor’s Section:
Medical Information
Therapeutic Group: ANTIBIOTICS
Sector: HUMAN
Composition:
Each vial contains:
Sterile Ceftriaxone Sodium 1gm + Sulbactam Sodium 500mg
Indication:
Bacterial meningitis, Bacterial septicemia,Surgical prophylaxis,Sexually transmitted diseases,Respiratory tract infections,Urinary tract infections,Serious skin and soft tissue infections,Pelvic inflammatory diseases, Infective endocarditis, Osteomyelitis.
Presentation & Pack:
Individual Vial
Doctor’s Section:
Medical Information

Composition
Dosage
Benefits
Precautions
Pack
Uses

Kapila Nature’s Gold Organic Foliar Bio-Stimulant Spray is for foliar application, specially developed to enhance health and growth potential of plants during critical stages like vegetative growth, flowering, seed and fruit formation.
Applications:
Vegetables, Fruit Trees, Plantation, Agricultural crops, Nurseries, flower beds, tree care, Lowns etc.
Benefits:
Contents:
Humic Acid, Fulvic Acid, Natural Gibberellins.
Chilli, Neem and clove Extracts, other ingredients
Extracts of
Dosage:
250 ml per acre for all crops in two spilt sprays. 250 ml to be mixed in 200 Its. of water before spraying at the time of active vegetative growth and repeat same dosage at the time of fruiting. Compatible with pesticides.
Available in: 100 ml, 250 ml, 1 litre and 5 litre packing.

Cypermethrin 10% EC High Cis Liquid
Therapeutic Group : Ectoparasiticide
Sector : VETERINARY
Each ml contains:
Cypermethrin 100 mg
To kill Ticks, Lice, Mites, Flies & Fleas effectively on the skin of the animals / birds as well as farm premises.
3.375 gm vial with 20 ml WFI and 20 ml syringe.
Dilution rate and method of application :
Farm animals : 0.5 ml to 1 ml per lit of water as wash or spray
Dogs : 0.5 ml to 1 ml per lit of water as wash or spray
Poultry : 1 ml per lit of water as 60 lits spray solution for 1000 birds.
Animal or Poultry Shed : 20 ml per lit of water as 5 lit water solution per 100Sq.meter.
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Each 1 gm Vial Contains:
Cefoperazone 500 mg + Sulbactum 500 mg.
Each 1.5 gm Vial Contains:
Cefoperazone 1 gm + Sulbactum 500 mg.
Injection 1 gm / 1.5 gm : Vial
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Each Vial Contains:
Ampicillin Sodium BP
equivalent to Ampicillin 250 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Each Vial Contains: Ampicillin Trihydrate 125 mg / 5 ml

Therapeutic Group : ANTITHROMBOTIC
Sector : HUMAN
Each Vial Contains:
Clopidogrel 75 mg
Aspirin 75 mg
• Primary & Secondary MI
• Unstable Angina
• Peripheral Arterial Disease
• Strokes
• Stent Thrombosis
Tablet : 3 x 10’s Strips in a carton
Therapeutic Group : ANTIHYPERTENSIVE / CARDIOVASCULAR
Sector : HUMAN
Composition: Atenolol 50 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:Benzathine Penicillin 6 lac units

Therapeutic Group : ANTITHROMBOTIC
Sector : HUMAN
Each tablet contains:
Clopidogrel 75 mg
• Primary & Secondary MI
• Unstable Angina
• Peripheral Arterial Disease
• Strokes
• Stent Thrombosis
Tablet : 3 x 10’s Strips in a carton
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:Benzathine Penicillin 12 lac units
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:Benzyl Penicillin 10 lac units
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:Benzyl Penicillin 5 lac units
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains: Benzyl Penicillin Sodium BP 3 g (5,000,000 units)
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains: Benzyl Penicillin Sodium BP 300 mg (5,00,000 units)
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:Benzyl Penicillin Sodium BP 600 mg (1,000,000 units)
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Capreomycin Sulphate BP equivalent to Capreomycin 1 g
Therapeutic Group : MULTIVITAMINS PLUS LACTIC ACID BACILLUS
Sector : HUMAN
Each capsule contains:
Lactic Acid Bacillus 120 million spores
Folic Acid 1.5 mg
Cyanocobalamin 15 mcg
• Adjuvant to antibiotics
• Diarrhoea & Dysentery
• Malabsorptive conditions
10 x 10’s Strips in a carton
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Cefaperazone 1 g (as Cefaperazone Sodium USP)
Sulbactam 1 g (as Sulbactam Sodium USP)

Therapeutic Group : MULTIVITAMINS PLUS LACTIC ACID BACILLUS
Sector : HUMAN
Each dispersible tablet contains:
Lactic Acid Bacillus 120 million spores
Folic Acid 1.5 mg
Cyanacobalamin 15 mcg
• Adjuvant to antibiotics
• Diarrhoea & Dysentery
• Malabsorptive conditions
Dispersible Tablets : 10 x 10’s Strips in a carton
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Cefaperazone 1 g (as Cefaperazone Sodium USP)
Sulbactam 1 g (as Sulbactam Sodium USP)
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:Cefaperazone 1 g (as Cefaperazone Sodium USP)
Therapeutic Group : ANTIBIOTIC
Composition:
Each vial contains:Cefaperazone 500 mg (as Cefaperazone Sodium USP)
Supplementing probiotics with prebiotics, which are agents that stimulate the growth and / or activity of nonpathogenic bacteria, is more beneficial than administering only probiotics.
Therapeutic Group : PRE AND PROBIOTIC
Clinical applications of probiotics in GI conditions
1. Diarrhoeas of various origins
2. Antibiotic associated diarrhoea
3. Rotavirus diarrhoea
4. Traveler’s diarrhoea
5. Gastroenteritis
6. Nosocomial diarrhoea
7. Clostridium difficile diarrhoea
8. HIV/AIDS diarrhoea
9. Enteral feeding-associated diarrhoea
Presentation & Pack:
Strips of 10 X 10’s caps in Alu – Alu pack
The recommended dosage
Adults : 1 – 2 Caps per day
Therapeutic Group : ANTIBIOTIC
Each vial contains
Cefotaxime sodium USP
equivalent to Cefotaxime 1 G
Therapeutic Group : ANTIDIARRHOEAL / ANTIDYSENTERIC
Sector : HUMAN
Each 5 ml contains:
Holarrhena Antidysentrica 200 mg
Punica Granatum 75 mg
Aegle Mermelos 20 mg
• Diarrhoea & Dysentery
• Irritable Bowel Syndrome
Syrup : 100 ml Bottle in a carton
Therapeutic Group : ANTIBIOTIC
Each vial contains
Cefotaxime sodium USP
equivalent to Cefotaxime 500 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:Cefotaxime Sodium 1.0 gm

Therapeutic Group : ANTIDIARRHOEAL / ANTIDYSENTERIC
Sector : HUMAN
Each tablet contains:
Holarrhena Antidysentrica 200 mg
Punica Granatum 100 mg
Aegle Mermelos 40 mg
• Diarrhoea & Dysentery
• Irritable Bowel Syndrome
Tablet : 30’s Container
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:Cefotaxime Sodium 500 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:Ceftazidime USP 1 g (as ceftazidime pentahydrate in a blend of sterile ceftazidime pentahydrate and sterile sodium carbonate ) eqivalent to Ceftazidime 1 G
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:Ceftazidime USP 2 G (as ceftazidime pentahydrate in a blend of sterile ceftazidime pentahydrate and sterile sodium carbonate ) eqivalent to Ceftazidime 2 G
Therapeutic Group : DIGESTIVE / LIVER -STIMULANT
Sector : HUMAN
Each tablet contains:
Piccrorhiza Kurroa 40 mg
Phyllanthus Niruri 75 mg
Tecomella Undulata 50 mg
Tephrosia Purpurea 100 mg
Eclipta Alba 80 mg
Boerrhavia Diffusa 25 mg
Azadirachta Indica 30 mg
• Infective Hepatitis
• Cirrhosis of Liver
• Digestive disorders
• Drug induced Hepatitis
Tablet : 100’s Container
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:Ceftazidime USP 250 mg (as ceftazidime pentahydrate in a blend of sterile ceftazidime pentahydrate and sterile sodium carbonate ) eqivalent to Ceftazidime 500 mg
Therapeutic Group : DIGESTIVE / LIVER -STIMULANT
Sector : HUMAN
Each 5 ml contains:
Piccrorhiza Kurroa 20 mg
Phyllanthus Niruri 30 mg
Tecomella Undulata 20 mg
Tephrosia Purpurea 50 mg
Eclipta Alba 50 mg
Boerrhavia Diffusa 20 mg
• Infective Hepatitis
• Cirrhosis of Liver
• Digestive disorders
• Drug induced Hepatitis
Syrup : 100 ml in a bottle
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:Ceftazidime USP 500 mg (as ceftazidime pentahydrate in a blend of sterile ceftazidime pentahydrate and sterile sodium carbonate ) eqivalent to Ceftazidime 500 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Ceftriaxone sodium USP
equivalent to Ceftriaxone 1 G
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Ceftriaxone sodium USP
equivalent to Ceftriaxone 250 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Ceftriaxone sodium USP
equivalent to Ceftriaxone 500 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Cefuroxime sodium USP
equivalent to Cefuroxime 1.5 G
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Cefuroxime sodium USP
equivalent to Cefuroxime 750 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each capsule contains: Chloramphenicol BP 250 mg
Therapeutic Group : ANTIFUNGAL / ANTIBACTERIAL
Sector : HUMAN
Each tube contains:
Beclomethasone Dipropionate 0.025 % w/w
Clotrimazole 1% w/w
Neomycin sulphate 0.5 % w/w
Iodo-Chlorhydroxyquinoline 1 % w/w
• Mixed fungal & bacterial infections
• Eczematous Mycosis
• Intertrigo
• Vulvo vaginitis
Cream : 10 gm Lamitube in a carton
To be applied sparingly 2-3 times daily on the affected area.
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:Chloramphenicol Sodium Succinate 1 gm
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Chloramphenicol sodium succinate BP
equivalent to 1.0 gm of Chloramphenicol
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition: Ciprofloxacin HCl 250 mg
Therapeutic Group : ANTIEMETIC / ANTIPYRETIC
Sector : HUMAN
Each 5 ml contains:
Domperidone 5 mg
Paracetamol 250 mg
• Pyrexia associated with nausea and vomiting
• Childhood migraine
Suspension : 60 ml Bottle in a carton

Therapeutic Group : ANTIMIGRAINE
Sector : HUMAN
Each tablet contains:
Domperidone 20 mg
Paracetamol 500 mg
• Acute migraine attack
• Tension headache
• Menstrual & Pre-menstrual cephalalgia
• Pyrexia associated with nausea and vomiting
Tablets : 10 x 10’s Strips in a carton
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition: Ciprofloxacin HCl 500 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition: Cloxacillin Sodium 250 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition: Cloxacillin Sodium 500 mg

Therapeutic Group : ANTIMIGRAINE
Sector : HUMAN
contains:
Each Tablet contains :
Flunarizine HCL 5 & 10 mg
Grenil F is recommended in :
Prophylaxis of migraine
Prophylaxis of vertigo & vestibular disorders
Prophylaxis of pheripheral & cerebrovascular disorders
Grenil – F 5 mg : Strips of 10 X 10’s in Blister pack
Grenil – F 10 mg : Strips of 10 X 10’s in Blister pack
The recommended dosage is as follows :
Aduts : 10mg once a day at bedtime.
7-15 Years / <40 kg : 5mg initially & 10mg later, depending on response
Doctor’s Section: Medical Information
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each capsule contains:
Cloxacillin 250 mg (as Cloxacillin Sodium BP)
Ampicillin 250 mg (as Ampicillin Trihydrate BP)

Therapeutic Group : BOWEL REGULATOR
Sector : HUMAN
contains:
Ispaghulla husk powder (Psyllium husk) 64.80%
Nimbu satwa (Citric acid) 10.00%
Sarijka kshar (Sodium bicarbonate) 8.52%
Chronic constipation.
Irritable bowel syndrome.
Haemorrhoids and Fissures.
Hypercholesteraemia & Obesity
Husky Granules is available in 100 gm container.
Husky Granules is available in 5 gm sachet
One teaspoonful once or twice daily along with plenty of water
Doctor’s Section:
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Cloxacillin sodium BP
equivalent to 250 mg of Cloxacillin
Ampicillin sodium BP
equivalent to 250 mg of Ampicillin
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Cloxacillin sodium BP
equivalent to Cloxacillin 250 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Cloxacillin sodium BP
equivalent to Cloxacillin 1 g
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Cloxacillin sodium BP
equivalent to Cloxacillin 500 mg
Therapeutic Group : ANTIINFECTIVE
Sector : HUMAN
Composition:
Each tablet contains:
Sulphamethoxazole 800 mg
Trimethoprim 160 mg
Therapeutic Group : ANTIINFECTIVE
Sector : HUMAN
Composition:
Each tablet contains:
Sulphamethoxazole 400 mg
Trimethoprim 80 mg
Therapeutic Group : ANTIINFECTIVE
Sector : HUMAN
Composition:
Each 5ml contains:
Sulphamethoxazole 200 mg
Trimethoprim 40 mg
Therapeutic Group : ANTIBACTERIALS
Sector : HUMAN
Composition:
Each uncoated tablet contains:
Trimethoprim BP 160 mg
Sulphamethoxazole BP 800 mg
Therapeutic Group : ANTIBACTERIALS
Sector : HUMAN
Composition:
Each Uncoated tablet contains:
Trimethoprim BP 80 mg
Sulphamethoxazole BP 400 mg
Therapeutic Group : STEROID ANTIINFLAMMATORY
Sector : HUMAN
Composition:
Each ml contains:
Dexamethasone Soidum Phosphate IP
equivalent to Dexamethasone 4 mg
Indication:
Presentation & Pack: Presented as 2 ml vial.
Therapeutic Group : STEROID ANTIINFLAMMATORY
Sector : HUMAN
Composition:
Each ml contains:
Dexamethasone Phosphate 4 mg (as Dexamethasone Sodium Phosphate USP)
Therapeutic Group : ANALGESIC / ANTIINFLAMMATORY
Sector : HUMAN
Composition: Diclofenac Sodium Inj 75 mg / 3ml
Therapeutic Group : ANTIARTHRITIC / ANTIINFLAMMATORY
Sector : HUMAN
Composition: Diclofenac Sodium 50 mg

Therapeutic Group : ANALGESIC
Sector : HUMAN
Each ampoule contains:
KAMADOL-50: Tramadol Hydrochloride 50 mg per ml
KAMADOL-100: Tramadol Hydrochloride 100 mg per 2 ml
• General, Orthopaedic & Gynaecological Surgery
• Maxillofacial & Other Dental Surgery
• Painful Bone Metastasis
• Acute Joint Pains
• Neuropathic Pain Syndromes
50 mg Injection : 1 ml Amp in 5’s carton
100 mg Injection : 2 ml Amp in 5’s carton
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition: Erythromycin Estolate 250 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each film coated tablet contains:
Erythromycin Stearate BP
equivalent to 250 mg of Erythromycin base
Therapeutic Group : HAEMOSTAT / CARDIOVASCULAR
Sector : HUMAN
Composition:
Each ml contains:
Etamsylate BP 125 mg
Water for Inj BP q.s
Therapeutic Group: ANTITUBERCULOSIS
Sector : HUMAN
Composition:
Each Uncoated tablet contains:
Ethambutol HCl BP 400 mg
Therapeutic Group : ANTITUBERCULOSIS
Sector : HUMAN
Composition:
Each Uncoated tablet contains:
Ethambutol HCl BP 800 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition: Fortified Procaine Penicillin 20 lac units
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition: Fortified Procaine Penicillin 4 lac units
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Procaine Benzyl penicillin BP 3 gm (3 mega units, )
Benzyl penicillin sodium BP 600 mg (1 mega units)
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Procaine Benzyl Penicillin BP 1.5 gm (1.5 mega units)
Benzyl penicillin Sodium BP 300 mg (0.5 mega units.)
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial contains:
Procaine Benzyl penicillin BP 300mg (0.3mega units)
Benzylpenicillin sodium BP 60mg (0.1 mega units)
Therapeutic Group :DIURETIC
Sector : HUMAN
Composition:
Each ml contains: Frusemide BP 10 mg
Therapeutic Group : ANTIPROTOZOAL / AMOEBICIDAL
Sector : HUMAN
Composition:
Each uncoated tablet contains: Furazolidone BP 100 mg
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition: Gentamicin Sulphate 80 mg / 2 ml
Indication:
Presentation & Pack: Presented as 2 ml both Vial and Ampoule.
Therapeutic Group : ANTIBIOTIC
Sector : HUMAN
Composition:
Each vial/ampoule contains:
Gentamicin 80 mg as Gentamicin Sulphate B.P
Therapeutic Group : ANTIHYPERTENSIVE
Sector : HUMAN
Each film coated tablet contains:
Losartan potassium 25 / 50 mg
Indicated in Mild and Moderate hypertension
Presented as blister pack of 10 X 10 tablets
Usually 1 tablet three times a day
Therapeutic Group : ANTIHYPERTENSIVE
Sector : HUMAN
Each film coated tablet contains:
Losartan potassium 50 mg
Hydrochlorothiazide 12.5 mg
Recommended in Moderate and Severe essential hypertension.
Presented as blister pack of 10 X 10 tablets
Usually 1 tablet two times daily.
Therapeutic Group : ANTIARTHRITIC / ANTIINFLAMMATORY / ANALGESIC
Sector : HUMAN
Numol MR 4 – ACECLOFENAC 100mg + THIOCOLCHICOSIDE 4mg TABLETS
Numol MR 8 – ACECLOFENAC 100mg + THIOCOLCHICOSIDE 8mg TABLETS
Degenerative vertebral disorders
Torticollis (Wry neck)
Dorsal pain and low back pain
Neurological disorders
NUMOL – MR 4mg & 8mg Tablets – Strips of 10×10’s in blister packs
Numol MR 4 – twice a day for 5 to 7 days
Numol MR 8 – once a day for 5 to 7 days
Therapeutic Group : ANTIINFLAMMATORY
Sector : HUMAN
Each tube contains:
Oleum Lini 3 % w/w
Diclofenac Diethylamine 1.16% w/w
Methyl Salicylate 10 % w/w
Menthol 5 % w/w
• Sports Injury
• Sprains & Strains
• Thrombophlebitis
• Physiotherapy
30 gm Lami Tube in a carton
Therapeutic Group : COUGH & COLD PREPARATION
Sector : HUMAN
Each 5 ml contains:
Paracetamol 125 mg
Chlorpheniramine Maleate 0.5 mg
Phenylephrine HCl 5 mg
Sod.Citrate 60 mg
Menthol 1 mg
• Allergic rhinitis
• Allergic Conjuctivitis
• Pruritus
• Urticaria
60 ml Bottle with a measuring cup

Therapeutic Group : COUGH & COLD PREPARATION
Sector : HUMAN
Each 5ml of REM-CC Expectorant contains:
Ambroxol Hydrochloride 30 mg
Terbutaline Sulphate 1.5 mg
Guiaphenesin 50 mg
Menthol flavoured syrupy base QS
• Acute & Chronic asthma.
• General & Smoker’s cough.
• As an adjuvant in : Upper & lower RTI.Sinusitis & congestive condition.
Bottle of 60 ml with a measuring cup.
Children : 2.5 ml – 5 ml 3 – 4 times daily.
Adults : 5 ml – 10 ml 3 – 4 times daily.
Therapeutic Group : ANTIHISTAMINIC / ANTIALLERGIC
Sector : HUMAN
Each tablet of RemCC LM contains:
Levocetirizine Hydrochloride – 5 mg
Montelukast Sodium – 10 mg
Seasonal Allergic Rhinitis
Perennial Allergic Rhinitis
Asthmatic conditions
Tablets : Box of 10×10’s tablets in blister strips.
Tablets:
Adults : 1 tablet once daily (at evening)
Therapeutic Group : COUGH & COLD PREPARATION
Sector : HUMAN
Each tablet contains:
Paracetamol 500 mg
Caffeine 30 mg
Phenylephrine Hcl 5 mg
Diphenhydramine Hcl 25 mg
• Allergic rhinitis
• Allergic Conjuctivitis
• Pruritus
• Urticaria
10 x 10’s Strips in a carton
Therapeutic Group: STEROID ANTIINFLAMMATORY
Sector : HUMAN
Composition:
Each vial contains:
Hydrocortisone 250 mg (as Hydrocortisone sodium succinate USP in a suitable buffer)
Therapeutic Group : STEROID ANTIINFLAMMATORY
Sector : HUMAN
Composition:
Each vial contains:
Kanamicin Acid sulphate BP
equivalent to Kanamycin 1 g
Therapeutic Group: STEROID ANTIINFLAMMATORY
Sector: HUMAN
Composition: Metronidazole 200 mg
Therapeutic Group: ANTIPROTOZOAL / AMOEBICIDAL
Sector: HUMAN
Composition:
Each uncoated tablet contains:
Metronidazole BP 200 mg
Therapeutic Group: ANTIPROTOZOAL / AMOEBICIDAL
Sector: HUMAN
Composition:
Each uncoated tablet contains:
Paracetamol BP 500 mg
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Procaine Benzyl Penicillin BP 1.0g (1 mega units)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Procaine Benzyl Penicillin BP 3.0g (3 mega units)
Therapeutic Group: ANTITUBERCULOSIS
Sector: HUMAN
Composition: Rifampicin 450 mg
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains: Sterile Penicillin G Benzathine USP 2.4 mega units (Appr. 1.8 g)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains: Sterile Penicillin G Benzathine USP 0.6 mega units (Appr. 450mg)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains: Sterile Penicillin G Benzathine U.S.P. 1.2 mega units (Appr. 900 mg)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition: Streptomycin Sulphate 0.75 mg
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition: Streptomycin Sulphate 1.0 mg
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Streptomycin sulphate BP
equivalent to 0.75 gm of Streptomycin.
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Streptomycin Sulphate BP
equivalent to 1 gm of Streptomycin.
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each capsule contains: Tetracycline HCl BP 250 mg
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each capsule contains: Tetracycline HCl 250 mg
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition: Tetracycline HCl 500 mg
Therapeutic Group: ANALGESIC
Sector: HUMAN
Composition:
Each ml contains:
Tramadol Hydrochloride 50 mg Water for Inj BP … q.s.
Therapeutic Group: ANALGESIC
Sector: HUMAN
Composition:
Each ml contains:
Tramadol Hydrochloride 50 mg Water for Inj BP … q.s.
Therapeutic Group: ANTIBACTERIALS
Sector: HUMAN
Composition:
Each vial of Cetriax – S contains :
Sterile Ceftriaxone Sodium 1 gm + Sulbactam Sodium 500 mg.
Indication:
Presentation & Pack:
Cetriax 1.0 gm – Vials containing Ceftriaxone Sodium 1 gm + sulbactuam sodium 500mg with 10 ml sterile water for
injection, in a shock
proof tray packed in individual carton.
Dosage:
Adults : The usual dose is 1 to 2 gm given once a day or in 2 eqully divided doses twice a day depending on the type and
severity of infection. The total daily dose should not exceed 4 gm.
Children :
Skin and soft tissue infections, 50 – 75 mg / kg / day given once a day or in 2 equally divided doses in a day. The total
dose should not exceed 2 gm. For serious infections other than meningitis, the daily dose is 50 to 75 mg / kg in divided
doses every 12 hours. Total daily dose should not be more than 2 gm. For meningitis a daily dose of 100 mg / kg limited
to a maximum of 4 gm / day is given in divided doses every 12 hours. No dose modification is needed in patients with
renal or hepatic impairment.
Doctor’s Section:

Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains :
Clavulanate Potassium 200 mg
Amoxycillin Sodium 1000 mg
Indication:
Recommended in infections like :
• URT / LRT
• Genito-Urinary
• Bone and soft tissue
• Pre and Post operative infections.
Presentation & Pack:
Presented as single unit vial pack in a carton
Dosage:
It is recommended only for IV injection only.
Adults – Usually 1.2 g every eight hours
Children – Usually 30 mg / kg every eight hours
Infants – Usually 30 mg / kg every twelve hours
Doctor’s Section: Medical Information

Therapeutic Group: ANTIPEPTIC ULCERANT
Sector: HUMAN
Composition:
Each capsule of Toprazol D contains : Pantoprazole (EC) 40 mg + Domperidone (SR) 30 mg
Indication:
Presentation & Pack:
Toprazol D capsules are available in blister pack of 10 x 10’s.
Dosage:
It is given orally in a dose of one capsule once daily about half an hour before breakfast.
Doctor’s Section:

Therapeutic Group: ANTIULCER / PROTON PUMP INHIBITORS
Sector: HUMAN
Composition:
Each tablet contains:Pantoprazole 40 mg
Indication:
Presentation & Pack:
10 x 10’s Strips in a carton
Dosage:
It is given orally in a dose of one capsule once daily about half an hour before breakfast.
Doctor’s Section: Medical Information
Therapeutic Group: COUGH & COLD PREPARATION
Sector: HUMAN
Composition:
Each 5 ml contains:
Ocimum Sanctum 100 mg
Sida Cordifolia 20 mg
Inula Racemosa 50 mg
Glycyrrhiza Glabra 50 mg
Zinziber Officinale 15 mg
Piper Longum 15 mg
Albizzia Lebbeck 50 mg
Adhatoda Vasaka 50 mg
Navasara 80 mg
Indication:
Presentation & Pack:
100 ml Bottle in a carton
Doctor’s Section: Medical Information
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each Tablet contains: Amoxycillin – 500mg Plus Clavulanate Potassium 125 mg
Dry Syrup : Each 5ml contains Amoxycillin 200mg plus Clavulanic acid 28.5mg
Indication:
Bacterial meningitis
Peritonitis
Post-operative infections
Genito-Urinary infections
LRTI- bronchitis
UTI
Skin & soft tissue infection
Enteric fever
Presentation & Pack:
Verclav 625 mgTab – 10×10 Alu Alu pack
Verclav Dry Syrup – 30ml with Carton
Dosage:
Adults- 250-500 mg every 8hr/ 500-750 mg every 12 hr
Children – 125-250 mg every 8 hr, if less than <40 kg: 20-40 mg/kg/day
Therapeutic Group: ANTIOSTEOPOROSIS
Sector: HUMAN
Composition:
Each softgel Capsule contains:
Calcitriol – 0.25mcg
Calcium Carbonate – 250mg
Zinc Sulphate – 7.5mg
Indication:
Postmenopausal osteoporosis
Senile osteoporosis
Corticosteroid induced osteoporosis
Presentation & Pack:
Vitatrol Plus is available in soft gelatin capsules in blister strips of 10 X 10’s .
Dosage:
The recommended dosage is one capsule twice daily. Serum Calcium levels are monitored frequently
Doctor’s Section: Medical information

Therapeutic Group: HAEMATINIC WITH ZINC AND VITAMINS
Sector: HUMAN
Composition:
Each tablet contains:
Ferrous bisglycinate
equivalent to elemental Iron 60 mg
Zinc bisglycinate
equivalent to elemental Zinc 15 mg
Folic acid 1 mg
Vitamin B 12 15 mcg
Each 5 ml of syrup contains:
Ferrous Bisglycinate -75 mg
equivalent to elemental Iron 15 mg
Folic acid I.P. 0.25 mg
Vitamin B12 I.P. 3.75 mcg
Zinc Picolinate- 5.5 mg
Flavoured Syrup base Q.S
Colour : Camosine
Appropriate overages of Vitamins added to compensate loss on storage.
Indication:
Zinfe – XT is indicated in pregnancy anaemia, nutritional anaemia, parasitic anaemia, Zinc deficiency and in post
operative cases as a supplement.Presentation & Pack:
Tablets: Strips of 3 X 10’s tablets in blister pack in a carton box.
Syrup: Bottle of 150 ml with measuring cup in a carton.
Dosage:
Tablet: One tablet once or twice a day.
Syrup: 2 teaspoonful once or twice a day.
Doctor’s Section: Medical Information
Therapeutic Group: HAEMATINIC WITH ZINC AND VITAMINS
Sector: HUMAN
Composition:
Each capsule contains:
Ferrous Sulphate 150 mg (in Sustained Release form)
Zinc Sulphate 61.8 mg (in Sustained Release form)
Folic Acid 1.5 mg
Cyanocobalamin 15 mcg
Pyridoxine HCl 3 mg
Indication:
Presentation & Pack:
10 x 10’s Strips in a carton
Dosage:
Capsule: One capsule once or twice a day.
Doctor’s Section: Medical Information
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains: Amikacin 100 mg (as Amikacin Sulphate USP)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:Amikacin 250 mg (as Amikacin Sulphate USP)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:Amikacin 500 mg (as Amikacin Sulphate USP)
Indication:Antibacterial
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Amoxicillin 250 mg (as Amoxicilin Sodium BP)
Cloxacillin 250 mg (as Cloxacillin Sodium BP)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Amoxicillin 500 mg (as Amoxicilin Sodium BP)
Cloxacillin 500 mg (as Cloxacillin Sodium BP)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each capsule contains: Amoxicillin 250 mg (as Amoxicillin Trihydrate BP)
Indication: Antibacterial
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each capsule contains: Amoxicillin 250 mg (as Amoxicillin Trihydrate BP)Each vial contains:
Amoxicillin sodium BP equivalent to 1 g of Amoxicillin
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Amoxicillin sodium BP
equivalent to 500 mg of Amoxicillin
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition: Amoxicillin Trihydrate 250 mg
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each capsule contains: Amoxicillin 250 mg (as Amoxicillin Trihydrate BP)Each vial contains:
Amoxicillin sodium BP equivalent to 1 g of Amoxicillin
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Amoxicillin sodium BP
equivalent to 500 mg of Amoxicillin
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition: Amoxicillin Trihydrate 250 mg
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Ampicillin 250 mg (as Amoxicilin Sodium BP)
Sulbactam 125 mg (as Sulbactam Sodium BP)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Ampicillin 500 mg (as Amoxicilin Sodium BP)
Sulbactam 250 mg (as Sulbactam Sodium BP)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each capsule contains:
Ampicillin 250 mg (as Ampicillin Trihydrate BP)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each capsule contains:
Ampicillin 500 mg (as Ampicillin Trihydrate BP)
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:Ampicillin Sodium 250 mg
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition: Ampicillin Sodium 500 mg
Therapeutic Group: ANTIBIOTIC
Sector: HUMAN
Composition:
Each vial contains:
Ampicillin Sodium BP
equivalent to Ampicillin 1 G
Therapeutic Group : ANTIBIOTIC
Sector : VETERINARY
Vials containing 500 mg:
2 gm and 3 gm Ceftriaxone
Sodium USP
Mastitis, Uro-
genital infections, Pneumonia, Post operative complications, Surgical wounds, Septic Wounds, Skin& soft tissue infections
500 mg, 2 gm and 3 gm vials.
Cow, Buffalo: 5-10 mg / Kg body weight
Calf, Sheep & Goat : 10-15 mg / Kg body weight
Dog & Cat: 15-25 mg / kg body weight
Duration: 3-5 days
Route of administration : I/M or I/V or as recommended by a registered veterinary practitioner
Ceftriaxone + Tazobactum Injection
Therapeutic Group : ANTIBIOTIC
Sector : VETERINARY
Each Vial Contains:
Ceftriaxone I.P : 3 gm
Tazobactum : 375 mg
1. Mastitis
2. Uro-genital infections
3. Pnemonia
4. Post operative complications
5. Wounds – ( Septic / surgical )
6. Skin and soft tissue infections
3.375 gm vial with 20 ml WFI and 20 ml syringe.
5-10 mg per kg body weight for 3-5 days through I/M or I/V route.
Ciprofloxacin + Tinidazole
Therapeutic Group : ANTIDIARRHOEAL / ANTIDYSENTERIC
Sector : VETERINARY
Each bolus contains:
Ciprofloxacin – 1500 mg
Tinidazole – 1800 mg
1. Purexia of unknown origin
2. Scours / diarrhoea / dysentery
3. Pnemonia
4. Navel ill
5. Wounds / Abscess
6. Geneto-urinary tract infections
Bolus : Carton of 2’s x 10 strips
Tablet : Box of 10’s x 10 strips
Bolus : 1 bolus per 300 kg body weight twice daily
Tablet : 1 tablet for 15-25 kg body weight twice daily.
Therapeutic Group : ANTHELMINTICS
Sector : VETERINARY
Each uncoated dispersible Bolus contains:
Fenbendazole – 1.5 gm
Each Tablet contains:
Fenbendazole 150 mg.
A Superior Broad Spectrum Anthelmintic with high margin of Safety.
Livestock: Highly effective against both mature &
immature stages of Gastro-intestinal Roundworms,
Tapeworms, Flukes, Covering Haemonchus, Ostertagia,
Trichostrongylus, Nematodirus, Dictyocaulus.
Horses: It is recommended in Strongyles, Ascarids and Oxyuris.
Dogs: Hook worms & Ascarids. Safe in Pregnancy.
Fenzole DB 1.5 gm –
Box of 5 x 2’s strip
Fenzole Tablet –
Box of 10 x 10’s strip.
Horses / Cattle / Pig: 1.5 gm /
300 kg bodyweight.
Dog: 50 mg / kg bodywieght or as recommended by the Veterinarian

Therapeutic Group : ANTIBACTERIALS
Sector : VETERINARY
Each ml contains:
Fenbendazole BP 25 mg.
A Superior Broad Spectrum Anthelmintic with high margin of Safety.
Livestock: Highly effective against
both mature & immature stages of Gastro-intestinal
Roundworms, Tapeworms, Flukes, Covering
Haemonchus, Ostertagia, Trichostrongylus,
Nematodirus, Dictyocaulus, Filaria.
Horses: It is recommended in Strongyles,
Ascarids and Oxyuris.
Dogs: Hook worms & Ascarids. Safe in Pregnancy.
100 ml, 500 ml and
1 Litre Bottle
LA:
6 ml suspension / 30 kg
bodyweight. 60 ml suspension /
300 kg bodyweight.
Dog: 50 mg / kg bodywieght or as
recommended by the Veterinarian.

Herbal remedy for milk let-down
Therapeutic Group : GROWTH PROMOTERS
Sector : VETERINARY
Each tablet contains:
Leptadenia reticulata – 200 mg
Glycyrrhiza glabra – 60 mg
Asparagus racemosus – 60 mg
Withania somnifera – 60 mg
Allium sativum – 10 mg
Trigonella foneumgracium – 10 mg
Hypogalactia, Agalactia, Irregular lactation,
Failure of lactation, Low milk production by
healthy cows / Buffaloes.
100 Tablets container
Bovines:
10 Tablets twice daily two hours prior to milking
Sheep & Goats:
2-3 Tablets per animal per day prior to milking

Therapeutic Group : ANTHELMINTICS
Sector : VETERINARY
Each tablet contains:
Kalbend tablet contains
Albendazole 150 mg.
Kalbend Bolus contains
Albendazole 600 mg.
Kalbend Forte Bolus contains
Albendazole 1.5 gm.
Livestock :
Broad Spectrum
Anthelmintic, effective against mature and
immature stages of Roundworm, Tapeworm,
Lungworm, Hookworm and Flukes.
Kalbend tablet –
Box of 10 x 10’s strip
Kalbend Bolus –
Box of 5 x 4’s strip
Kalbend Forte Bolus –
Box of 5 x 2’s strip
Worm dose:
Livestock: 5 mg / kg bodyweight.
Fluke dose:
Cattle: 10 mg / kg bodyweight.
Sheep / Goat: 7.5 mg / kg
bodyweight or as recommended
by the Veterinarian

Vitamins & Minerals powdered feed supplement for poultry
Therapeutic Group : Vitamins & Minerals Mixture
Sector : Poultry
Each 5 kg contains:
Viatmin A 100,00,000 IU
Vitamin B 2 6 gm
Vitamin B 6 2 gm
Vitamin B 12 20 mcg
Vitamin D 3 20,00,000 IU
Vitamin E 1600 IU
Vitamin K 2.0 gm
Calcium pantothenate 6 gm
Nicotinic acid 20 gm
Calcium 1600 gm
Phosphorus 100 gm
Manganese 60 gm
Iodine 2 gm
Iron 16 gm
Zinc 40 gm
Copper 5 gm
Cobalt 1 gm
Choline chloride 300 gm
Anti-oxidants added to prevent oxidation of vitamins.
1. To optimize FCR
2. To maximize the growth rate of birds
3. To optimize egg yield & Meat yield of birds
4. To alleviate stress and related disorders
5. To enhance Immunity of birds
2.5 kg & 5 kg bag
Breeders : 5 kg per tonne of feed
Growers / layers : 2.5 kg per tonne of feed
Therapeutic Group : GROWTH PROMOTERS
Sector : VETERINARY
Each 2.5 kg contains:
Kalvimin Forte Contains:
Vitamin A – 50 lacs IU
Vitamin B 2 – 2.0 gm
Vitamin B 12 – 6.0 mcg
Vitamin D 3 – 10 lacs IU
Vitamin E – 800 IU
Calcium Pantothenate – 4.0 gm
Calcium – 800 gm
Phosphorous – 150 gm
Manganese – 27.5 gm
Iodine – 1.0 gm
Iron – 7.5 gm
Zinc – 15.0 gm
Copper – 2.0 gm.
Anti-oxidants added to prevent
oxidation of vitamins.
1. To Optimize Growth & Health of calves and heifers
2. To alleviate stress and related disorders
3. To enhance immunity of animals
4.To enhance fertility of animals
5.To boost the milk production in lactating animals
6. To boost the milk production in lactating animals
Box of 10 x 100 gm pouch
1 kg bag &
2.5 kg Bag
2.5 kg per tonne of cattle feed

Metho-Chelated vitamins and mineral mixtures enriched with Bypass fat, Essential amino acids, live yeast and bioactive chromium.
Therapeutic Group : Vitamins & Minerals Mixture
Sector : VETERINARY
Each kg contains:
Calcium 260 gms
Phosphorous 130 gms
Magnesium 6 gms
Manganese 1.5 gms
Iron 1.5 gms
Iodine 325 gms
Copper 4200 mg
Zinc 9600 mg
Cobalt 150 mg
Sulphur 7.2 gms
Pottasium 100 mg
Sodium 5.9 mg
Selenium 10 mg
Vitamin A 7,00,000 IU
Vitamin D 3 70,000 IU
Vitamin E 250 mg
Nicotinamide 1 gm
Biotin 550 mcg
Bypass fat 20 gms
Lactobacillus sporogenes 15 x 10 10 CFU
Sacchromyces cerevisiae 3 x 10 10 CFU
Bioactive chromium 65 mg
DL Methionine 1920 mg
L-Lysine 4400 mg
1. Anoestrus
2. Under developed genetalia
3. Scanty oestrus discharge
4. Delayed maturity
5. Repeat breeding
6. Poor growth rate
7. Low milk yield with poor SNF and Fat content.
1 kg and 5 kg bag
Cow & Buffalo : 30-35 gms per head per day
Sheep & Goat : 10-15 gms per head per day
Pigs : 30-35 gms per head per day
Aqua : 1 kg per 100 kg of feed.

Vitamin concentrate liquid feed supplement for poultry
Therapeutic Group : MULTIVITAMINS
Sector : Poultry
Each ml contains:
Vitamin A 12000 IU
Vitamin D 3 6000 IU
Vitamin E 48 mg
Vitamin B 12 20 mcg
Methionine 2 mg
Zinc 3 mg
1. To optimize growth & Health of birds
2. To alleviate stress and related disorders
3. To enhance immunity of birds.
30 ml, 60 ml & 500 ml
Chicks & Growers : 5 ml / 100 birds / day
Broilers & Layers : 10 ml / 100 birds / day
Duration : 5-7 days
Good growth…better weight….best profits
Enrofloxacin injection 10% w/v
Therapeutic Group : ANTIBACTERIALS
Sector : VETERINARY
Each ml contains:
Enrofloxacin 100 mg
A broad spectrum antibacterial.
Livestock:
Livestock: Effective against E.Coli,
Salmonella Spp., Moraxella bovis,
Hemophilus Spp., Pseudomonas aeruginosa,
Brucella canis. Staphylococcus Spp.,
Streptococcus Spp., Corynebacterium pyogenes,
Clostridium perfringens, Erysipelothrix,
Mycoplasma Spp., Leptospira Spp.,
Poultry: Mycoplasmosis, Coli-septicaemia,
CRD, Coryza, Fowlcholera, Salmonellosis, Arthritis,
Erysipelas, Staphylococcus. Also in mixed infections
& secondary bacterial complications in GI tract,
Urinary Tract, se
15 ml, 30 ml and
50 ml vials.
In cattle / Buffalo / Sheep / Goat / Camel / Pigs:
5 mg / kg bodyweight for 3-5 days.
In Poultry:
1 ml to be diluted
with 9 ml distilled water & given
0.5 ml / kg bodyweight.
Deep intramuscular injection
should be administered
at 24 hours interval or as
recommended by the Veterinarian

Oral electrolytes formula for poultry
Therapeutic Group : Electrolytes
Sector : Poultry
Each 100 gm contains:
Sodium chloride 0.8 gm
Potassium chloride 5 gm
Sodium bicarbonate 3 gm
Sodium citrate 1.75 gm
Calcium lactate 1.75 gm
Magnesium sulphate 1 gm
Ascorbic acid 500 mg
Lactobacillus spores 3000 million
Glycine 125 mg
Dextrose 60 gm
Manganese sulphate 500 mg
Carrier Q.S
1. To alleviate heat stress in birds
2. To alleviate production stress in laying birds
3. To alleviate stress during & after transportation
4. As a supportive therapy for birds during convalescence period.
250 gm pouch & 1 kg pack
Water Mixing Ratio:
1 gm / 2 lits of water for 3-5 days
Feed Mixing Ratio:
1 kg / tonne of poultry feed.
Rehydrate & Rejuvenate

The Complete Liver Tonic
Therapeutic Group : LIQUID FEED SUPPLEMENT
Sector : VETERINARY
Each 5 ml contains:
Tricholine Citrate – 700 mg
Vitamin B 12 – 10 mcg
Niacinamide – 15 mg
Inositol – 12 mg
Biotin – 10 mcg
DL-Methionine 5 mg
base enriched with extract of
liver and yeast.
1. To prevent & Treat “Fatty Liver Syndrome” in Livestock & Poultry
2. To optimize liver function
3. To regenerate liver cells
4. To correct anorexia and improve appetite
5. To improve milk yield
200 ml, 500 ml,
1000 ml bottle &
5 litres Can
Cattle:
25-50 ml per day/animal
Sheep & Goat
5-10 ml per day/animal
Poultry:
Chicks: 5-10 ml/100 birds/day
Growers & Layers: 15-20 ml/100 birds / day
Breeders: 50-100 ml/100 birds/day
Duration: 5-7 days

Poultry Liver protector feed Premix
Therapeutic Group : DIGESTIVE / LIVER -STIMULANT
Sector : Poultry
Hepato protectors, Mannan Olego Saccharides ( MOS ), Amino acids & Phosphatides
1. To mobilize fat from liver and prevent “Fatty Liver Hemorrhagic Syndrome”
2. To regenerate liver cells
3. To optimize liver function
4. To enhance growth rate, Egg Yield and Meat Yield
5. To increase body weight gain & FCR through better digestibility.
1 kg and 25 kg bag
Broilers : 500 gm to 1 kg / tonne of feed
Layers : 500 gm / tonne of feed
Breeders : 1-1.5 kg / tonne of feed
Healthy liver…..healthy birds
Therapeutic Group : ANTIBACTERIALS
Sector : VETERINARY
Kloxamp 2 gm vial contains:
Ampicillin Sodium I.P. 1 gm &
Cloxacillin Sodium I.P. 1 gm.
Kloxamp 3 gm vial contains:
Ampicillin Sodium I.P. 1.5 gm &
Cloxacillin Sodium I.P. 1.5 gm.
Kloxamp 4 gm vial contains:
Ampicillin Sodium I.P. 2.0 gm
Cloxacillin Sodium I.P.2.0 gm
Livestock: Anthrax, BQ, HS,
Pneumonia, Bronchitis, Nephritis, Mastitis,
Metritis, Cystitis, wounds & Abscess .
2 gm,
3 gm & 4 gm vial
6 to 10 mg / kg bodyweight
daily for 3 to 5 days. I/M or I/V
injection or as recommended
by the Veterinarian
Therapeutic Group : ANTHELMINTICS
Sector : VETERINARY
Each bolus contains:
Oxyclozanide IP (Vet) 1000 mg,
Levamisole Hcl IP (Vet) 500 mg
Nematodes,Paramphistomiasis, Amphistomes, Fasciola (liver fluke) and Mixed infestations.
Immuno-potentiating action of Levamisole is an added advantage.
Box of 5 x 4’s strip
Livestock: 10 -15 mg / kg
bodyweight or 1 bolus / 100 kg
bodyweight orally or as
recommended by the Veterinarian

Oxyclozanide + Levamisole HCL combined dewormer
Therapeutic Group : ANTHELMINTICS
Sector : VETERINARY
Each ml contains:
Oxyclozanide I.P 3 % w/v
Levamisole HCL I.P 1.5 % w/v
1. To Kill all stages of round worm in farm animals.
2. To kill liver flukes and amphistomes more effectively.
3. To enhance the Immunity status of the animals.
100 ml and 1 Litre.
10-15 mg per kg body weight or 90-100 ml per 300 kg body weight orally as drench.

CB-Complex vitamins, amino acids and minerals
Therapeutic Group : Vitamins & Minerals Mixture
Sector : Poultry
Each 50 gm contains:
Vitamin B 1 500 mg
Vitamin B 2 250 mg
Vitamin B 6 130 mg
Vitamin B 12 1200 mcg
Niacinamide 7000 mg
D-Panthenol 250 mg
Lysine 1000 mg
Methionine 500 mg
Copper 75 mg
Cobalt 10 mg
Zinc 40 mg
Iron 40 mg
Manganese 50 mg
Iodine 5 mg
1. To optimize growth & Health of birds
2. To increase the livability of chicks
3. To alleviate stress and related disorders.
4. To optimize feather/Plumage growth
5. To prevent Nutritional disorders of birds.
50 gm pouch
Chicks : 10 gm / 2000 chicks / day
Broilers : 20 gm / 2000 birds / day
Growers : 20 gm / 2000 birds / day
Layers : 10 gm / 2000 layers / day
Duration : 3-5 days.
Alleviate stress…..optimize livability


A unique blend of enzymes & trace minerals
Therapeutic Group : GROWTH PROMOTERS
Sector : VETERINARY
Contains:
Cellulase, Xylanase, Pectinase,
Phytase and Trace Minerals
1. To Maximize “Feed utilization” by animals.
2.To Optimize
A. Fibre Digestion
B. Milk Yield
C. Milk Fat & SNF
3. To control wet litter droppings in poultry
100 gm pouch, 300 gm
and 500 gm HDPE
container & 5 kg bag
Cattle Feed Mixing Ratio:
1 Kg per tonne of feed
Large animals:
10-20 gm per animal per day
Small animals:
5 gm per day per animal
Therapeutic Group : ANTIBACTERIALS
Sector : VETERINARY
Each 3 gm vial contains:
Amoxycillin Sodium I.P 2.0 gm
Sulbactum Sodium U.S.P. 1 gm
Pneumonia, Lower respiratory tract
infection, Upper respiratory tract infection, Mastitis,
Bone & soft tissue infection
3 gm vial
with Water for Injection
7 mg per kg body weight per day
I/V or I/M or as recommended
by the veterinarian.
Therapeutic Group : ANTIBACTERIALS
Sector : VETERINARY
Each Vial Contains:
Procaine Benzyl Penicillin IP
15,00,000 units
Benzyl Penicillin Sodium IP
5,00,000 units
Streptomycin Sulphate IP 2.5 gm
As a broad spectrum Antibiotic highly effective
against both Gr+ve & Gr-ve bacterial infections and ,
mixed-bacterial Infections, RTI, UTI,
Peritonitis, infected wounds etc
2.5 gm vial
Preparation: To each vial add
7.5 ml of sterile distilled water
to make 10 ml suspension
Large Animals : 2 ml / 50 kg bodyweight.
Small Animals: 1 ml / 5 kg bodyweight.
I/M route only or as
recommended by the Veterinarian
Therapeutic Group : ANTISPASMODICS
Sector : VETERINARY
Each ml contains:
Dicyclomine Hydrochloride IP 10 mg.
Recommended in all types of Spasmodic &
Visceral pains of Gastro-intestinal tract, Renal,
Billiary, Uterus. Prolapse of Vagina,
Urinary Bladder, Rectal, Recto-Vaginal, Uterine.
Pains associated with functional Diarrhoea,
Irritable Bowel Syndrome & Distocia. In non specific
abdominal & visceral pains.
30 ml vial
LA:
10-15 ml, SA: 0.5-2 ml, by
I/M route only. May be repeated
after 12 hours if needed.
Contra Indications:
not recommended in new born &
premature animals, debilitated &
weak animals, old animals,
in Pyloric / Duodenal obstruction &
Urinary retention cases or as
recommended by the Veterinarian
Therapeutic Group : ANTIBACTERIALS
Sector : VETERINARY
Each vial contains:
Streptomycin Sulphate I.P. 5 gm.
Pasteurellosis, Brucellosis, Salmonellosis and
in diseases caused by Hemophilus, Klebsiella,
Shigella and Mycobacterium organisms.
5 gm vial.
LA:
11 mg per kg bodyweight I/M
route only at intervals of 8 to 12 hrs.
Poultry: 44-99 mg / kg bodyweight
I/M route only or as recommended
by the Veterinarian
Therapeutic Group : ANTIBACTERIALS
Sector : VETERINARY
Each bolus Contains:
Sulphadimidine IP 5 gm.
Livestock:
Septicemia, Pneumonia,
Foot rot, Bronchitis, Scour, Bacterial diarrhoea,
Enteritis, Coccidiosis and Genito-urinary tract
infections like Metritis, Retention of Placenta etc.
Box of 5 x 4’s strip
LA:
1 to 2 bolus / 50 kg
bodyweight daily.
SA:
1 bolus / 25 kg bodyweight
daily (followed by half of the dose).
Appetite Stimulant & Hepato-protective bolus
Therapeutic Group : STOMACHIC & DIGESTIVE HERBAL TONIC
Sector : VETERINARY
Each 5 gm Bolus contains:
Chitraka – 100 mg, Pippali – 50 mg,
Sunti – 100 mg,
Mareecha – 100 mg,
Hingu – 50 mg,
Ajavana – 175 mg,
Shankha Bhasma – 250 mg
Bringaraj – 250 mg
Boomiamla – 750 mg
Guduchi – 250 mg
Haritaki – 500 mg
Kakamchi – 590 mg
Dyspepsia,Anorexia, Indigestion, Constipation, Flatulence, Gastro-Intestinal tract disorders, Drug/Toxin induced
liver damage, supportive therapy in Jaundice & Hepatitis, co treatment with anthelmintics and antibiotic, and as a
General health tonic
Box of 10x 4’s strip.
Cow,Buffaloe & Horse:
1-2 boli orally twice daily for 5 days.
Calf,Colt,Pig,Sheep & Goat:
Half bolus orally once daily for 5 days.

Herbal Rumenotoric Powder
Therapeutic Group : STOMACHIC & DIGESTIVE HERBAL TONIC
Sector : VETERINARY
Each 5 gm Powder contains:
Chitraka – 2 gm
Pippali – 200 mg
Sunti – 200 mg
Mareecha – 200 mg
Hingu – 350 mg
Ajavana – 550 mg
Shankha Bhasma – 1500 mg
Anorexia, Indigestion, Constipation, Flatulence,
Gastro-Intestinal tract disorders and as a
General health tonic
100 gm, 200 gm & 1 Kg
Cow / Buffalo / Horse:
25-50 gms twice daily for 5 days.
Calf / Colt / Pig / Sheep / Goat:
10-15 gm once daily for 5 days.
Mixed with treacle or made
electuary and administered orally
or as recommended by the
Veterinarian.
Piperazine liquid 45% w/v
Therapeutic Group: ANTHELMINTICS
Sector: Poultry
Composition:
Each 5 ml contains: Piperazine hydrate 2.25 gms
Indication:
To kill larger round worms in Poultry
Presentation & Pack:
30 ml, 500 ml and 5 Litre can
Dosage:
500 ml / 2000 birds below 6 week
500 ml / 1000 birds above 6 week
Doctor’s Section:
Freedom from Ascarid Worms


Meloxicam + Paracetamol injection
Therapeutic Group: ANALGESIC / ANTIINFLAMMATORY
Sector: VETERINARY
Composition:
Each ml contains:
Meloxicam I.P 5 mg
Paracetamol I.P 150 mg
Benzyl alcohol 2% w/v
Water for injection I.P QS
Indication:
Presentation & Pack:
30 ml, 50 ml and 100 ml
Dosage:
Cow, Buffalo, Sheep, Goat, Pig & Horse : 1 ml per 10 kg body weight through I/M route of administration
Doctor’s Section: Freedom from fever and pain
Meloxicam + Paracetamol bolus
Therapeutic Group: ANALGESIC / ANTIINFLAMMATORY
Sector: VETERINARY
Composition:
Each bolus contains:
Meloxicam 100 mg
Paracetamol 1500 mg
Indication:
Presentation & Pack:
Pack of 10 x 4’s boli
Dosage:
Large animals: 1-2 boli per day per animal per oral
Small animals : Half bolus to one bolus per day per animal per oral.
Doctor’s Section: Freedom from fever & pain
Meloxicam + Paracetamol bolus
Therapeutic Group: ANTIBACTERIALS
Sector: VETERINARY
Composition:
Each bolus contains:
Pyrexia of unidendified, origin, diarrhoea, Pnemonia, HS, BQ, Actinomycosis, NavelIll, Actinobacillosis, wounds, Abscess, Metritis, ROP etc.
Indication:
Presentation & Pack:
Box of 5 x 2’s strip
Dosage:
Large animals:
2 to 3 Bolus / day for
3 to 5 days orally.
As Intra-Uterine Pessary:
1⁄2 to 1 bolus directly or
as douche, dissolved in water.
Small Animals:
1⁄2 to 1Bolus / day orally
or as recommended by the
Veterinarian.

Therapeutic Group : ANTIBACTERIALS
Sector : AGRO
Each 6 gm pouch contains:
Streptomycin Sulphate 90 % IP
Tetracycline Hydrochloride 10 % IP
(100% technical ingredients)
• Non-Pollutant.
• Protects the crops from all the bacterial diseases.
• Extremely active against phytopathogenic bacteria and effectively controls various bacterial diseases of different crops.
Available in 6 gms and 500gms
• 6 gms x10 pouches in a carton
30 X10 cartons in master pack.
• 500 gms X 8 containers in a master pack
2-3 sprays at 15 to 20 days interval is recommended. Depending upon climatic conditions and disease intensity, the interval between two sprays may be fixed. Products to be mixed with water before spray, quantity of water depends upon the crops and diseases with different method of application.
Therapeutic Group :Antidiarrhoeal, Anti dysenteric.
Sector : AGRO
Dadima (Punica granatum ) 75 mg.
Bilva (Aegle marmelos ) 20 mg.
Kutaja (Holarrhena antidysenterica) 200 mg.
Jatiphala (Myristicafragrans ) 10 mg.
Sweta Jeeraka (Cuminum cyminum ) 10 mg.
Shunthi (Zingiber officinale ) 5 mg.
Excipients q.s.
Sodium Methyl Paraben I.P. q.s.
Sodium Propyl Paraben I.P. q.s.
Bronopol I.P q.s.
Colour : Sunset Yellow FCF Supra.
• Antidiarrhoeal.
• Digestive.
• Anti dysenteric.
10 X 10 Tablets
Doctor’s Section:
Therapeutic Group : Antacid.
YastiMadhu ( Glycyrrhiza Glabra ) 150 mg.
Amalaki ( Emblica officinalis ) 150 mg.
Narikela lavana (Classical preparation) 100 mg.
Shankha Bhasma ( Classical preparation) 100 mg.
Excipients q.s.
Sodium Methyl Paraben I.P. q.s.
Sodium Propyl Paraben I.P. q.s.
Sodium Benzoate I.P. q.s.
Colour : Tartrazine Supra
• Antacid.
• Gastric ulcer.
• Duodenal ulcer.
10 X 10 Tablets
Therapeutic Group : Hepatoprotective
Sharapunkha (Tephrosia purpurea) 100 mg.
Bhringaraja (Eclipta alba ) 80 mg.
Bhoomyamalaki (Phyllanthus niruri ) 75 mg.
Rohitaka (Tecomella undulata) 50 mg.
Katuki (Picrorrhiza kurroea) 40 mg.
Nimba (Azadirachta indica ) 30 mg.
Punarnava ( Boerrhavia diffusa ) 25 mg.
Excipients q.s.
Sodium Methyl Paraben I.P. q.s.
Sodium Propyl Paraben I.P. q.s.
Sodium Benzoate I.P q.s.
Colour : Pronceau 4R Supra.
• Hepatoprotective.
• Jaundice.
10 X 10 Blister pack
Doctor’s Section:
Therapeutic Group : Renal calculus.
Pashanabedha (Berginia ligulata) 120 mg.
Varuna (Cretavia Nurvula) 60 mg.
Gokshura (Tribulus terrestris) 60 mg.
Punurnava (Boerrhavia diffusa) 60 mg.
Yavakshara (Hordeum vulgare) 50 mg.
Hazanuyahood Bhasma 25 mg.
• Renal calculus.
10 X 10 Blister pack
Therapeutic Group : Laxative
Sonamukhi (Cassia angustifolia) 250 mg.
Shunti (Zinzeber officinale) 20 mg.
Ajamoda (Phytochis ajowan) 25 mg.
Hingu (Ferula asafoetida) 20 mg.
Triphala 50 mg.
• Laxative.
10 X 10 Blister pack
Therapeutic Group : Menstural Regularisation
Ashoka (Saraca indica) 150 mg. Lodhra (Symplocos recemosus) 100 mg.
Jeeraka (Cuminum cyminum) 25 mg.
Shatavari (Asparagus racemosus ) 50 mg.
Guduchi (Tinospora cordifolia ) 25 mg.
Dhanyaka (Coriandrum Sativum) 25 mg.
Triphala 50 mg.
Shilajit purified 25 mg.
• Leucorrhoea.
10 X 10 Blister pack
Therapeutic Group : Cough & Cold Preperation
Anthrapachaka (Tylophora Asthmatica) 150 mg.
Vasaka (Adathoda vasika ) 50 mg.
Yastimadhu (Glycyrrhiza glabra ) 25 mg.
Tulsi (Ocimum sanctum ) 50 mg.
Pippali (Piper longum ) 50 mg.
Somalatha (Ephedra vulgaris ) 25 mg.
Bala (Sida cordifolia ) 50 mg.
Karpura ( Cinnamomum camphora ) 5 mg.
Haridra ( Curcuma longa ) 20 mg.
Kantakari (Solanum xanthocarpum) 50 mg.
• Bronchial Asthma.
10 X 10 Blister pack
Therapeutic Group : Piles Remedy
Guggulu (Commiphora mukul) 50 mg.
Sallaki (Boswella serrata) 100 mg.
Haridra (Curcuma longa) 20 mg.
Kanchanahara (Bauhimia variegata) 50 mg.
Lajjalu (Mimosa pudica) 50 mg.
Sonamukhi (Casia angustifolia) 50 mg.
Daruharidra (Berberis aristata ) 25 mg.
Triphala 50 mg.
• Hemorrhoids,
• All types of piles.
10 X 10 Blister pack
Therapeutic Group : Joint Pain Management
Guggulu (Commiphora Mukul) 100 mg.
Sallaki (Boswelia Sererata ) 100 mg.
Haridra (Curcuma longa) 20 mg.
Rasna (Pluchea lanceolata ) 50 mg.
Nirgundi (Vitex negunda) 50 mg.
Shunti (Zingerber officinale) 50 mg.
Jeera (Cumminum cyminum) 25 mg.
Eranda (Ricinus communis) 25 mg.
• Rheumatic arthritis.
• Joint Pain.
10 X 10 Blister pack
Therapeutic Group : Cough & Cold Preperation
Guggulu (Commiphora Mukul) 100 mg. Haridra (Curcuma longa) 20 mg.
Pippali (Piper longum) 10 mg.
Nimba (Azardirachta indica) 50 mg.
Yashtimadhu (Glycyrrhiza glabra) 50 mg.
Methika (Trigonellafoenum graceum) 100 mg.
Daruharidra (Berberis aristata) 25 mg.
Triphala 50 mg.
Purified Shilajit 10 mg.
Gokshura ( Tribulus terrestris) 50 mg.
Tankana 25 mg.
• Respiratory Tract Infection.
• Immunobooster.
10 X 10 Blister pack
Therapeutic Group : Anti – diabetic.
Gudmar (Gymnema sylvestre ) 100 mg.
Haridra ( Curcuma Longa) 50 mg.
Nimba (Azadiracta indica ) 75 mg.
Karavellaka ( Momordica charantia 100 mg.
Methika(Trigonella foenumgraeum ) 50 mg.
Bilva (Aegle marmelos ) 50 mg.
Gokshura (Tribulus terrestris) 50 mg.
Triphala 50 mg.
Asana ( Peterocarpus marsupium) 50 mg.
SuddhaShilajit ( Mineral pitch Purified ) 10 mg.
• Anti – diabetic.
10 X 10 Blister pack
Therapeutic Group : Cardiac Tonic
Arjuna (Terminalia arjuna) 200 mg.
Triphala 50 mg.
Pravala bhasma (Incinerated Caco3) (Coral) 20 mg.
Akik bhasma (Incinerated ) 20 mg.
Abraka bhasma (Incinerated Mica) 20 mg.
Swarnamakshika bhasma (Incinerated ferric Sulphate) 20 mg.
Shilajit shuddha (Purified mineral pitch) 20 mg.
Loh bhasma (Incinerated iron ) 20 mg.
• Cardiac Tonic
10 X 10 Blister pack
Therapeutic Group : Anti-Hypertensive
Sarpagandha (Rauwolfia serpentine) 200 mg.
Shankhapushpi (convolvulus pluricaulis) 100 mg.
Brahmi (Bacopa monnieri ) 100 mg.
Ashwagandha (Withania somnifera) 100 mg.
• Anti-Hypertensive
• Anti-Stress.
10 X 10 Blister pack
Therapeutic Group : Cough & Cold Preperation
Katuki (Picrorhiza kurroa) 100 mg.
Haridra ( Curcuma longa) 50 mg.
Tulsi (Ocimum sanctum) 50 mg.
Giloy (Tinospora cordifolia) 50 mg.
Triphala 100 mg.
Trikatu 100 mg.
• Cold & Cough with Fever.
10 X 10 Blister pack
Therapeutic Group: Antipyretic.
Katuki (Picrorhiza kurroa) 200 mg.
Amrita (Tinospora cordifolia) 100 mg.
Tulsi (Ocimum sanctum ) 50 mg.
Triphala 100 mg.
Musta (Cyperus rotundus ) 50 mg.
Parpata ( Fumaria indica ) 50 mg.
• Antipyretic.
10 X 10 Blister pack

Therapeutic Group : Platelet Booster
Erand karkati (Carica papaya) 1100 mg.
• Dengue Fever

Therapeutic Group : Antidiarrhoeal. Anti dysenteric.
Dadima (Punica granatum ) 100 mg.
Bilva (Aegle marmelos ) 40 mg.
Kutaja (Holarrhena antidysenterica) 20 mg.
Jatiphala (Myristicafragrans ) 10 mg.
Sweta Jeeraka (Cuminum cyminum ) 10 mg.
Shunthi (Zingiber officinale ) 5 mg.
Excipients q.s.
Sodium Methyl Paraben I.P. q.s.
Sodium Propyl Paraben I.P. q.s.
Bronopol I.P. q.s.
• Antidiarrhoeal. Anti dysenteric.
• Digestive.
100 ml
Doctor’s Section:
Therapeutic Group : Antacid & anti flatulent.
Sharapunkha (Tephrosia purpurea) 50 mg.
Bhringaraja (Eclipta alba ) 50 mg.
Bhoomyamalaki (Phyllanthus niruri ) 30 mg.
Rohitaka (Tecomella undulata) 20 mg.
Kutaki (Picrorrhiza kurroa) 20 mg.
Punarnava (Boerrhavia diffusa ) 20 mg.
Excipients q.s.
Sodium Methyl Paraben I.P. q.s.
Sodium Propyl Paraben I.P. q.s.
Bronopol I.P q.s.
• Antacid & anti flatulent.
• Gastric & duodenal ulcer.
100 ml
Doctor’s Section:
Therapeutic Group : Cough & Cold Preperation
Tulsi (Ocimum Sanctum) 100 mg
Shodita Navarasa (Navasara) 80 mg.
Vasaka (Adhatoda Vasaka ) 50 mg.
Pushkaramoola (Inula Racemosa) 50 mg.
Shirish (Albizzia Lebbeck ) 50 mg.
Yastimadhu ( Glycyrrhiza Glabra) 50 mg.
Bala (Sida Cordifolia ) 20 mg.
Pippali (Piper Longum ) 15 mg.
Shunthi ( Zinziber Officinale ) 15 mg.
Excipients q.s.
Sodium Methyl Paraben I.P. q.s.
Sodium Propyl Paraben I.P. q.s.
Bronopol I.P. q.s.
• Cough Syrup.
• Expectorant.
• Allergic bronchitis.
100 ml
Doctor’s Section:
Therapeutic Group : Renal calculi
Pashanabedha (Berginia ligulata) 120 mg.
Varuna (Cretavia Nurvula) 60 mg.
Gokshura (Tribulus terrestris) 60 mg.
Punurnava (Boerrhavia diffusa) 60 mg.
Yavakshara (Hordeum vulgare) 50 mg.
Hazaluyahood Bhasma 15 mg.
• Renal calculus.
• Urinary / Kidney diseases.
100 ml

Therapeutic Group :Diuretic.
Gokshura (Tribulus terrestris) 50 mg.
Varuna (Cretaeva nurvula) 50 mg.
Sariva (Hemidesmus indica) 50 mg.
Ushira (Vettiveria zizanioides) 50 mg.
Shatavari (Asparagus racemosus) 50 mg.
Lajjalu (Mimosa pudica ) 20 mg.
Manjista (Rubia cordifolia) 10 mg.
Trikatu 10 mg.
Shilajit Purified 10 mg.
• Urinary alkaliser, Diatrutic.
• Diatrutic.
100 ml
Therapeutic Group : Cough & Cold Preperation
Anthrapachaka (Tylophora asthmatica) 50 mg.
Kantakari (Solanum xanthocarpum) 50 mg.
Vasaka (Adathoda vasika) 50 mg.
Yastimadhu (Glycyrrhiza glabra) 50 mg.
Tulsi (Ocimum sanctum) 50 mg.
Pippali (Piperlongum) 10 mg.
Somalatha (Ephedra vulgaris) 20 mg.
Bala (SidaCordifolia ) 50 mg
Karpura (Cinnamomumcamphora) 5 mg.
Haridra (Curcuma longa) 10 mg.
• Bronchial Asthma
100 ml

Therapeutic Group :Blood Purifier
Nimba (Azadirachta indica) 25 mg.
Guduchi (Tinosphora cordifolia) 25 mg.
Vidanga (Embelia ribes) 25 mg.
Kalmegh (Andrographis paniculata) 50 mg.
Haridra (Curcuma longa) 25 mg.
Aragvadha (Cassia fistula) 10 mg.
Bhringaraj (Eclipta Alba) 50 mg.
Bhumyamalki (Phyllanthus niruri) 25 mg.
•Blood Purifier
100 ml
Therapeutic Group : Antacid & Antiflatulent.
YastiMadhu (Glycyrrhiza glabra ) 150 mg.
Amalaki (Emblicaofficinalis ) 50 mg.
Nimba (Azadirachta indica) 10 mg.
Narikela Lavana (Classical preparation) 50 mg.
Shankha Bhasma (Classical preparation) 50 mg.
Excipients q.s.
Sodium Methyl Paraben I.P. q.s.
Sodium Propyl Paraben I.P. q.s.
Sodium Benzoate I.P. q.s.
• Antacid & antiflatulent.
100 ml
Therapeutic Group :Heamatinic syrup.
Amalaki (Embelica officinalis) 200 mg.
Shatavari (Asparagasracemosus) 100 mg.
Chandan (Santalum album) 50 mg.
Draksha (Vitis vinfera) 100 mg.
Khajur (Phenonin dactylfera) 100 mg.
Dashamoola 100 mg.
Loha Bhasma (Incinerated iron) 25 mg.
Mandura Bhasma (Ferric oxide) 25 mg.
• Heamatinic syrup.
100 ml

Therapeutic Group : Antipyretic.
Katuki (Picrorhiza kurroa) 200 mg.
Amrita (Tinospora cordifolia) 100 mg.
Tulsi (Ocimum Sanctum) 50 mg.
Triphala 100 mg.
Musta (Cyperus rotundus) 100 mg.
Parpata (Fumaria indica) 50 mg.
Draksha (Vitis vinifera) 100mg.
• Antipyretic.
100 ml

Therapeutic Group : Appetizer
Katuki (Picrorhiza kurroa) 30 mg.
Vidanga (Embelia ribes) 20 mg.
Musta (Cyperus rotundus ) 20 mg.
Pippali (Piper longum ) 30 mg.
Amalaki ( Emblica officinalis ) 100 mg.
Shunthi (Zingiber officinale ) 50 mg.
Dadima (Punica granatum ) 100 mg.
Draksha (Vitis vinfera) 30 mg.
Mishreya (Foeniculum vulgare) 50 mg.
Mentha Piperata (Puteeha) 50mg
• Appetizer
• Digestive
200 ml in a carton pack
http://kaplindia.com/apifeast-syp/

Therapeutic Group : Platelet Booster
Erand karkati (Carica papaya) 550 mg.
• Dengue Fever
150 ml in a carton pack

Therapeutic Group : Pain Management
Guggulu (Commiphora Mukul ) 2% w/v
Sallaki (Boswelia serrata) 2%
Nirgundi (Vitex negundo) 1%
Tailapatra (Eucalyptus globulus) 2%
Gandhapura Tail (Gaulheria fragrantissima) 2%
China karpoor (Chinnamomum camphora) 1%
Katuveera (Capsicum annum) 1%
Erand tail (Ricinus communis) 50% v/v
Til taila (sesamumIndicum) Q.S Base 50% v/v.
• Joint pain.
• Rheumatism.
50ml
Therapeutic Group : Pain Management
Puteeha (Mentha piperata) 5%
China Karpoor (Cinnamomum camphor) 2%
Tail Patra (Eucalyptus globulus) 2%
GanhaPuro (Gaultheria pragrantissima) 2%
Sarla (PinusLongifolia ) 1%
Katuveera (Capsicum Annum) 2%
• Joint pain.
• Rheumatism.
• Muscle relaxant.
20 gm
Therapeutic Group : An antiseptic cream.
Kumari (Aloe barbadensis) 5% w/w
Haridra (Curcumin longa) 3.25% w/w
Chandan (Santalum alba ) 3% v/w
Jati (Jasminum grandiflora) 3% v/w
Madhu 1% w/w
Cocunut oil to make it 100%
• An antiseptic cream.
• Usefull in burns,scars.
20 gm
Therapeutic Group :Antiinflamatory
Yastimadhu (Glycyrrhiza glabra ) 3% w/w
Babbula (Acacia Arabica) 3% w/w
Haridra (Curcuma Longa) 2% w/w
Lajjalu (Mimosa Pudica) 2% w/w
Lodhra (Symplocos racemosa) 2% w/w
Pippala (Ficus religiosa) 2% w/w
Vata (Ficus benghalensis) 2% w/w
Udumbara (Ficus glomerata) 2%.
Usheera (Vettiveria ziznoides) 1% w/w
Madhu (Honey) 1% w/w
TilTaila (Sesamum indicum) 15% v/w.
• Piles , Proctitis.
• Inflamatory condition.
20 gm
Therapeutic Group : Fungicidal
Nimba (Azadiracta indica) 6% v/w
Haridra (Curcuma longa) 3% w/w
Khadira (Acacia catechu) 3% w/w
Manjista (Rubia cordifolia) 3% w/w
Chandana (Santalum album) 1% v/w.
• Fungal infection.
20 gm
Therapeutic Group :Cough & Cold Preperation
Gandhapuro ( Gaultheria fragrantissima) 3%
Lavanga (Syzygium aromaticum) 1%
Katuveera (Capsicum annum) 1%
Puteeha (Mentha piperata ) 1.5%
Sarala (Pinus longifolia) 2%
Daarusita (Cinnamomum zeylanicum) 1.5%
Ajamoda (Apium graveolens) 2%
China Kapoor (Cinnamomum Camphor) 1%
Taila patra ( Eucalytus clobulus) 2.5%
• Cold,Headache.
• Rhinitis.
10 gm (Container pack)

Therapeutic Group: Laxative
Ispaghula 65%
Nimbu Satwa 12.22%
Sarjika Kshar 11.48%
Excipients q.s.
• Constipation.
• Irritable bowel syndrome
100 gm

Therapeutic Group: DE-WORMER
Each mL contains:
Flumethrin 10 mg
Solvents q.s.
To exterminate and control all type of ticks including ticks species resistant to carbamates, amides, chlorinated hydrocarbons, organophosphates and an organochlorine group of insecticides.
30 mL, 50 mL & 100 Ml
Apply with Fluvet – self-applicator bottle at a dose rate of
1 mL/10 kg body weight evenly on the midline of back from the front of the shoulder to the tail setting or as directed by the veterinarian.
Precautionary Measures:
Keep Fluvet out of reach of children. Avoid contact of the Fluvet solution with mucous membrane and skin. If any person makes contact with this solution accidentally, he needs to wash immediately and thoroughly with soap and water.
Therapeutic Group: DE-WORMER
Each ml contains
Ivermectin IP 10 mg
Features & Benefits
• Kills tick, lice, mite, flea, fly & mosquito on the skin of the animals
– Kills round worm, lung worm & eye worm more effectively
10 ml &50 ml
0 1 ml per 50 Kg body weight through Subcutaneous route

Therapeutic Group: DE-WORMER
Each ml contains
Closantel Sodium Dihydrate IP equivalent to Closantel 150 mg
Aqueous Base Q.S.
To treat and prevent animals suffering from roundworms, liver flukes, and ectoparasites infestation
30 ml & 500 ml containers
Flukes &Ectoparasites1ml per 10 kg body weight roundworms 1 ml per 16 kg body weight or as directed by the Veterinary Practitioners

Therapeutic Group: DE-WORMER
Each ml contains Oxyclozanide IP Levamisole HCL IP
Features & Benefits
3 % w/v 1.5 % w/v
• Proven Flukicide: Kills adult liver flukes more effectively
• Powerful Nemotocidal&Immunoptentiator: Kills roundworm & enhance the immunity status of farm animals
100 ml & 1 Liter 4) 5 x 4’s Boll
Farm animals: 90-100 ml per 300 Kg body weight at the dose rate of 10-15 mg per kg body weight orally as drench

Therapeutic Group: FEED SUPPLEMENT
Each kg contains
Organically Hydrated sodium calcium
• Aluminosilicate (HSCAS) 620 gm
• Sterilized activated charcoal 20 gm
• Acetic acid 15 gm
• Ammonium formate 15 gm
• Citric acid salt 10 gm
• Calcium propionate 20 gm
• Phosphorylated Mannan-oligosaccharide (MOS) 100 gm
• Bacillus subtilis 8000 million
• Sodium bentonite 193.2 gm
• Preservatives 0.5 gm
• Bentonite Q.S
To prevent and treat multiple mycotoxicoses in Livestock, Poultry & Aqua
25 kg of Paper Craft Bag
Regular use: 1 kg per tonne of feed
During bacterial toxins / contaminations: 2-3 kg per tonne of feed

Therapeutic Group: FEED SUPPLEMENT
Each bolus contains
Saccharomyces cerevisiae 25 x 109 CFU
Lactobacillus sporogenes 25 x 106 CFU
Aspergillus oryzae 25 x 10 6 CFU
Fructo oligosaccharide 250 mg
Biotin 10 mg
Methionine 1 gm
Zinc Sulphate 200 mg
Cobalt Sulphate 50 mg
Copper Sulphate 100 mg
Stabilizes rumen pH thus prevent acidosis Increases cellulolytic bacteria thus optimize fibre digestion Promotes growth thus ensure early maturity of calves Optimizes digestion thus better feed efficiency Controls methane emission thus environmental protection Optimizes milk yield
24 x 4’s strip
Large animals : 1-2 boli twice daily Small animals : 1/2 to 1 bolus twice daily

Therapeutic Group : HERBAL
Sector : VETERINARY
Cuminumcyminum (Jeera) 120 mg
Carumcopticum (Yavani) 120 mg
Cassia angustifolia (Senna) 120 mg
Ferula narthex (Hingu) 100 mg
Classical Preparation (ShankhBhasma) 100 mg
Ammonichloridum (ShudhNavshadar) 100 mg
Terminalia chebula (Haritaki) 60 mg
Terminalia bellirica (Vibhitaki) 60 mg
Emblicaofficinalis (Amalaki) 60 mg
Piper nigrum (Maricha) 50 mg
Piper longum (Pippali) 50 mg
Zingiberofficinale (Shunti) 50 mg
Asparagus racemosus (Shatavari) 50 mg
Sod. Benzoate (as preservative) q.s
Frothy bloat (primary tympany)
Free gas bloat (secondary tympany)
Flatulence
Abdominal colic (UdaraShoola)
100 ml pet bottle
Cattle / Buffalo: 100 ml orally once daily
Horse & Mule: 50-100 ml orally once daily
Sheep & Goat: 10-20 ml orally once daily
or as directed by veterinarian
Each bolus Contains:
Essential phospholipids 1000 mg
Tribasic calcium phosphate 3600 mg
Vitamin D3 8000 IU
VItamin B12 100 mcg
Cobalt chloride 100 mg
Leptadeniareticulata 920 mg
Nardostachysjatamansi 300 mg
Protein hydrolysate (20%) 600 mg
Colour Erythrosine
• To optimize and sustain milk production in
• lactating animals
• To optimize Milk Fat & SNF percentage
• To strengthen the fertility of farm animals
• To accelerate growth in young animal
5 X 4’s boli
Therapeutic Group: Blood Purifier
Nimba (Azadirachta indica) 25 mg.
Guduchi (Tinosphora cordifolia) 25 mg.
Vidanga (Embelia ribes) 25 mg.
Kalmegh (Andrographis paniculata) 50 mg.
Haridra (Curcuma longa) 25 mg.
Aragvadha (Cassia fistula) 10 mg.
Bhringaraj (Eclipta Alba) 50 mg.
Bhumyamalki (Phyllanthus niruri) 25 mg.
Blood Purifier
100 ml

Therapeutic Group: HERBAL
Each ml contains (Extracts & Distillates of)
Eucalyptus globulus (Eucalyptus) 63 mg
Azadirachta indica (Neem) 56 mg
Cymbopogon citratus (Lemon grass) 42 mg
Cedrus deodara (Devadaru) 30 mg
Curcuma longa (Turmeric) 8 mg
Pinus longifolia (Pine tree) 30 mg
Aseptic and septic wound • FMD wounds • Pox lesions Maggoted wounds • Surgical wounds Dermatomycosis Shearing wounds in sheep • Pruritis due to Sarcoptic, Psoroptic mange, Demodectic mange & Chorioptic mange Foot Rot Wing rot in poultry • Wounds associated with Yoke and Rope Gall • Lacerated Wounds Sores and Fissures Ringworm • Degnala
Directions for Use:
Shake well Kapcure Plus container Clean the wound with Kapcure plus soaked cotton Spray Kapcure Plus in adequate quantity twice daily on the wounds and other skin affections till complete recovery
100 ml Spray Can
Therapeutic Group: ANTIBACTERIALS
Each vial contains BENZYL PENICILLIN IP 50 LAKHS
Helps to treat deadly Bacterial infections
50 lakhs vial
8000 to 20000 IU per Kg body weight at 4hours interval through I/V or I/M route
Therapeutic Group: NSAID’S
Each ml contains
Meloxicam BP 5 mg
Arthritis, Osteoarthritis, Lameness, Sprain, Colic, Pneumonia, Bronchopneumonia, Mastitis, Laminitis, Myositis, Prolapse of Uterus & FMD
30 ml and 100 ml vials
• 30 ml / 300 kg body weight in Mastitis, Pneumonia & Prolapse of uterus
• 15 ml / 375 kg body weight for Arthritis, Sprain, Lameness & Myositis
• Horse – 30 ml / 250 kg body weight
• Sheep & Goat – 2 ml / 33 kg body weight I/M or I/V or as recommended by the Veterinarian.
Therapeutic Group: HORMONE
1. To stimulate uterine contractions for facilitating parturition in animals.
2. To accelerate involution of uterus in postpartum animals.
3. To use as a adjunct therapy in Retained Fetal Membranes (RFM)
4. To control and stop postpartum hemorrhages
5. To expel the pathological content of uterus in Endometritis and Pyometra
6. To promote Milk Let-down in case of Agalactia
7. To remove residual milk and toxic material from the udder after parturition and during the treatment of infectious mastitis in animals.
Target Species:
Bovine, Equine, Ovine, Caprine, Porcine and canine
Withdrawal Period:
Milk: 7 days
Meat: 28 days
• Clear liquid in 1 mL glass ampoules
• 25 labelled and blister packed ampoules in a printed carton
Special Warning:
• The treated animal should not be stressed as stress may reduce the effect of oxytocin.
• Exogenic oxytocin is not recommended as a control tool to increase the milk production.
• Pregnant women should avoid handling with this veterinary medicinal product.
Storage:
• Keep out of reach of children.
• Store at a temperature between 2-8* C
• Protect from Frost
Shelf Life:
• 2 years from the date of manufacturing.
• Partly used ampoules to be discarded immediately.
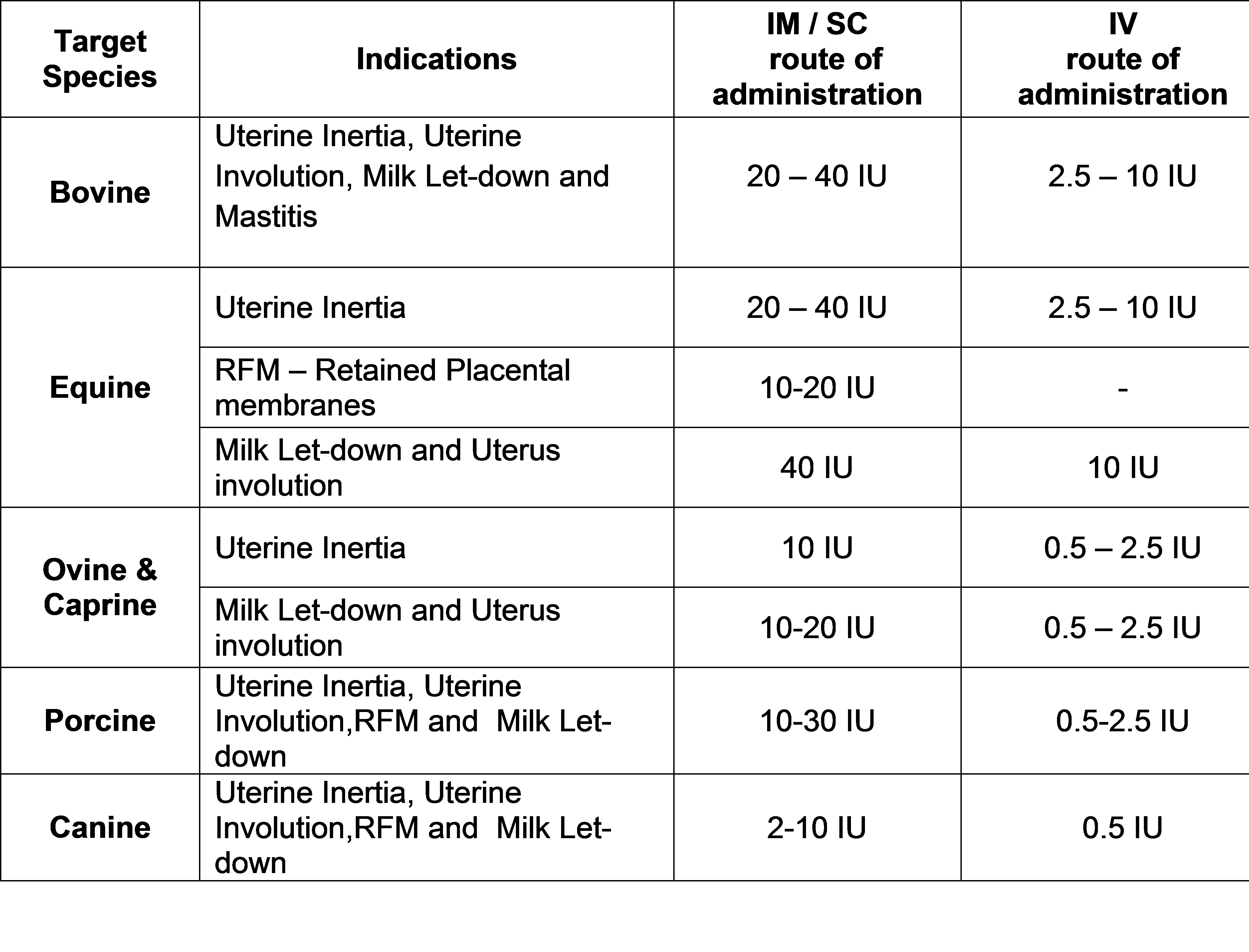
Therapeutic Group: ANTIBACTERIAL
4.0 gm / 60 ml container
Add 60 ml sterile water to 4.0 gm dry suspension and shake well and infuse intra uterine with the help of A.I. gun

Therapeutic Group: ANIMAL FEED SUPPLEMENT
Each 100 ml contains
Calcium 1750 mg
Phosphorus 875 mg
Vitamin D3 8000 IU
Vitamin B12 100 mcg
Vitamin E 100 IU
Carbohydrate Extracts of-Asparagus racemosus 1000 mg
Leptadeniareticulata 1000 mg
Piper longum 400 mg
Bioactive chromium 2 mg
• Optimizes Milk yield
• Optimizes Milk fat & SNF
• Optimizes Appetite & Digestion
• Optimizes reproductive organs health
• Optimizes Bone & Skeletal integrity
• Reduces the incidence of Ketosis,
• Milk Fever, ROP & Acidosis.
1 litre & 5 litre
Cow& Buffalo : 100 ml daily to Sheep & Goat : 10 ml daily w Calf & Heifer : 40 ml daily

Therapeutic Group: HERBAL
Each 10 ml Contains:
Saracaindica (Ashoka Tree) 1000 mg
Asparagus racemosus (Shatavari) 350 mg
Curculigoorchioides (Kali musali) 200 mg
Aegle marmelos (Bael tree) 150 mg
Symplocosracemosa (Lodh tree ) 150 mg
Semecarpusanacardium (Marking Nut) 250 mg
Rubiacordifolia (Indian Madder) 200 mg
Abromaaugusta (Devil’s cotton ) 150 mg
Commiphoramyrrha (Myrrh) 150 mg
Terminalia arjuna (Arjun tree ) 150 mg
Puerariatuberosa (Kudzu) 150 mg
Bhuteafrondosakoen (Palash) 150 mg
Berberisaristata (Daruharidra) 150 mg
Withaniasomnifera (Ashwagandha) 150 mg
Hemidesmusindicus (Sariva) 150 mg
Adhatodavasica (Malabar nut ) 150 mg
Convolvulus microphyllus (Shankapuspi) 150 mg
Acoruscalamus (Sweet flag ) 100 mg
Sod. Benzoate (as preservative)
• For expulsion of retained placenta
• To flush out lochial discharge
• For faster involution of uterus
• To prevent Endometritis, Metritis &Pyometra
500 ml pet bottle
Cow, Buffalo & Horse: 50 ml orally twice daily Sheep & Goat: 25 ml orally twice daily or as directed by veterinarian

Therapeutic Group: ANIMAL FEED SUPPLEMENT
Each bolus Contains:
Essential phospholipids 1000 mg
Tribasic calcium phosphate 3600 mg
Vitamin D3 8000 IU
VItamin B12 100 mcg
Cobalt chloride 100 mg
Leptadeniareticulata 920 mg
Nardostachysjatamansi 300 mg
Protein hydrolysate (20%) 600 mg
Colour Erythrosine
• To optimize and sustain milk production in
• lactating animals
• To optimize Milk Fat & SNF percentage
• To strengthen the fertility of farm animals
• To accelerate growth in young animal
5 X 4’s boli
Therapeutic Group: ANTIPROTOZOAL / AMOEBICIDAL
Each Tablet contains: Tinidazole IP 500 mg
Treatement of Bacterial Infections
10×10
Therapeutic Group: ANTIPROTOZOAL / AMOEBICIDAL
Each Tablet contains: Tinidazole IP 300 mg
Treatement of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Capsule contains: Tetracyclin IP 500 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Capsule contains: Tetracyclin IP 500 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Roxithromycin IP 150 mg
Treatement of Bacterial Infections
10×10
Therapeutic Group: ANTITUBERCULOSIS
Each capsule contains: Rifampin USP 450 mg
Therapeutic Group: ANTITUBERCULOSIS
Each capsule contains: Rifampin USP 300 mg
Therapeutic Group: ANTITUBERCULOSIS
Each capsule contains: Rifampin USP 150mg
Therapeutic Group: ANTIULCER / PROTON PUMP INHIBITORS
Each Tablet contains: Ranitidine 150 mg
Treatement of Gastro-Intestinal Disorders
10×10
Therapeutic Group: ANTIULCER / PROTON PUMP INHIBITORS
Each Ampoule contains: Ranitidine 25 mg/ ml
Treatment of Gastro-Intestinal Disorders
2ml Amp.
Therapeutic Group: ANALGESIC / ANTIINFLAMMATORY
Each Tablet contains: Aceclofenac IP 100 mg + Paracetamol 325 mg
(THE ITEM TO BE SUPPLIED AS PER DCGI OM F.NO.18-06/2011-DC DATED 04.04.2012 I.E ACECLOFENAC IP 100MG + PARACETAMOL IP 325MG )
Used for management of Pain
10×10
Therapeutic Group: ANTHELMINTICS
Each Tablet contains: Albendazole IP 400 mg (Chewable)
Mixed Worm Infestation
10×10
Therapeutic Group: ANTHELMINTICS
Each 5ml contains: Albendazole IP 200 mg
Mixed Worm Infestation
10 ml Bottle
Therapeutic Group: ANTIBIOTIC
Each 2 ml contains:Amikacin 100 mg
Treatement of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each 2 ml contains:Amikacin 250 mg
Treatement of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each vial contains:
Ampicillin Sodium BP
equivalent to Ampicillin 500 mg
Therapeutic Group: ANTIBIOTIC
Each 2 ml contains: Amikacin 500 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each 5ml contains: Amoxycillin IP 125 mg
Treatment of Bacterial Infections
60 ml Bottle
Therapeutic Group: ANTIBIOTIC
Each Capsule contains: Amoxycillin IP 250 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Capsule contains: Amoxycillin IP 500 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each vial contains: Amoxycillin Sodium 1000 mg+ Potassium Clavulanate 200 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each vial contains: Amoxycillin Sodium 250 mg +
Potassium Clavulanate 50 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each vial contains:
Cefazoline sodium USP
equivalent to Cefazoline 1 g
Therapeutic Group: ANTIBIOTIC
Each vial contains:
Cefazoline sodium USP
equivalent to Cefazoline 1 g
Therapeutic Group: ANTIBIOTIC
Each vial contains:
Cefazoline sodium USP
equivalent to Cefazoline 500 mg
Therapeutic Group: ANTIBIOTIC
Each vial contains: Amoxycillin Sodium 500 mg+
Potassium Clavulanate 100 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Capsule contains: Ampicillin IP 250 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each vial contains: Ampicillin IP 250 mg
Treatment of Bacterial Infections
Vile
Therapeutic Group: ANTIBIOTIC
Each Capsule contains: Ampicillin IP 500 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIHYPERTENSIVE / CARDIOVASCULAR
Each Tablet Contains: Atenolol IP 25 mg
Management of Hypertension
10×14
Therapeutic Group: ANTIHYPERTENSIVE / CARDIOVASCULAR
Each Tablet Contains: Atenolol IP 50 mg
Management of Hypertension
10×14
Therapeutic Group: ANTIHYPERTENSIVE / CARDIOVASCULAR
Each Tablet Contains: Atorvastatin IP 10 mg
Management of Caronary Artery Diseases
10×10
Therapeutic Group: ANTIHYPERTENSIVE / CARDIOVASCULAR
Each Tablet Contains: Atorvastatin IP 20 mg
Management of Caronary Artery Diseases
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet Contains: Azithromycin 250 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet Contains: Azithromycin 500 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each vial contains: Benzathene Penicillin Sodium IP 12 lac units
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each vial contains: Benzathene Penicillin Sodium IP 6 lac units
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIARTHRITIC / ANTIINFLAMMATORY / ANALGESIC
Each ml contains:
Diclofenac Sodium BP 25 mg
Therapeutic Group: Vitamins & Minerals Mixture
Each Tablet Contains: Calcium 250 mg +
Vitamin D3 125 I.U
Osteoporosis & Calcium Deficiency
10×10
Therapeutic Group: Vitamins & Minerals Mixture
Each Tablet Contains: Calcium 500 mg +
Vitamin D3 250 I.U
Osteoporosis & Calcium Deficiency
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: CefadroxIl IP 500 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Cefixime 100 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Cefixime 200 mg
Treatment of Bacterial Infections
3×10
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Cefoperazone 500 mg+ Sulbactum 500 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Cefoperazone 1000 mg +Sulbactum 1000 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Cefotaxime Sodium1.0 gm
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Cefotaxime Sodium 250 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Cefotaxime Sodium 500 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Cefotaxime Sodium 1gm + Sulbactum 500mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each vial contains:
Hydrocortisone 100 mg (as Hydrocortisone sodium succinate USP in a suitable buffer)
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Cefotaxime Sodium 250mg + Sulbactum 125mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Cefotaxime Sodium 500mg + Sulbactum 250mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each vial contains: Anhydrous Ceftazidime IP 250 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIDIABETIC
Each Tablet contains: Metformin IP 500 mg
Management of Diabetes Mellitus
10×10
Therapeutic Group: ANTIPROTOZOAL / AMOEBICIDAL
Each Tablet contains: Metronidazole 200 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIPROTOZOAL / AMOEBICIDAL
Each Tablet contains: Metronidazole 400 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIINFLAMMATORY
Each Tablet contains: Nimesulide IP 100 mg
Used for management of Pain
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Norfloxacin IP 400 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Norfloxacin IP 400 mg+ Tinidazole 600 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Ofloxacin IP 200 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Ofloxacin IP 200 mg + Ornidazole 500mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Ofloxacin IP 400 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIULCER / PROTON PUMP INHIBITORS
Each Capsule contains: Omeprazole IP 20 mg+ Domperidone 10 mg
Treatment of Gastro-Intestinal Disorders
10×10
Therapeutic Group: ANTIULCER / PROTON PUMP INHIBITORS
Each Capsule contains: Omeprazole IP 20 mg
Treatement of Gastro Intestinal Disorders
10×10
Therapeutic Group: ANTIDIARRHOEAL / ANTIDYSENTERIC
Each Sachet of 21 gms contains: Sodium Chloride I. P 2.6 gms, Potassium Chloride I. P 1.5 gms, Sodium Citrate I. P 2.9 gms,Dextrose (Anhydrous) I.P 13.5 gms, Excipients q.s
Management of Electrolyte loss during Diarrhoea
21 G Sachet
Therapeutic Group: ANTIINFLAMMATORY
Each Tablet contains: Paracetamol IP 325mg+Ibuprofen IP 400 mg
Used for management of Pain
10×10
Therapeutic Group: ANTIINFLAMMATORY
Each Tablet contains: Paracetamol IP 325mg+Diclofenac Sodium IP 50 mg
Used for management of Pain
10×10
Therapeutic Group: ANTIINFLAMMATORY
Each Tablet contains: Paracetamol IP 500 mg
Used for management of Pain
10×10
Therapeutic Group: ANTIINFLAMMATORY
Each 5ml contains: Paracetamol IP 125 mg
Used for management of Pai
60 ml Bottle
Therapeutic Group: ANTIBIOTIC
Each vial contains: Piperacillin 4 gm + Tazobactum 0.5 gm
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIINFLAMMATORY
Each Tablet contains: Ibuprofen 200 mg
Used for management of Pain
10×10
Therapeutic Group: ANTIINFLAMMATORY
Each Tablet contains: Ibuprofen 400 mg
Used for management of Pain
10×10
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Streptomycin 0.75 gm (As Streptomycin Sulphate. IP)
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Streptomycin 1.0 gm (As Streptomycin Sulphate. IP)
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each vial contains:
Kanamicin Acid sulphate BP
equivalent to Kanamycin 1 g
Therapeutic Group: ANTIALLERGIC
Each Tablet contains: Levocetrizine IP 5 mg
Treatment of Cold & Cough
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Levofloxacin IP 250 mg
Treatement of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Levofloxacin IP 500 mg
Treatement of Bacterial Infections
10×10
Therapeutic Group: ANTIHYPERTENSIVE / CARDIOVASCULAR
Each Tablet contains: Losartan Potassium 25 mg
Management of Hypertension
10×10
Therapeutic Group: ANTIHYPERTENSIVE / CARDIOVASCULAR
Each Tablet contains: Losartan Potassium 50mg
Management of Hypertension
10×10
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Meropenem IP 1gm
Treatement of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Meropenem IP 500 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Erythromycin IP 250 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Erythromycin IP 500 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTITUBERCULOSIS
Each Uncoated tablet contains:
Ethambutol HCl BP 400 mg
Therapeutic Group: ANTIFUNGAL / ANTIBACTERIAL
Each Capsule contains: Fluconazole 150 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: DIURETIC
Each Ampoule contains: Frusemide 10 mg/ ml
Diuresis
2ml Amp.
Therapeutic Group: DIURETIC
Each ml contains:
Frusemide BP 10 mg
Therapeutic Group: DIURETIC
Each uncoated tablet contains:
Frusemide BP 40 mg
Therapeutic Group: ANTIBIOTIC
Each 2ml Vial contains Gentamycin 80 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIDIABETIC
Each Tablet contains: Glimepiride 1mg
Management of Diabetes Mellitus
10×10
Therapeutic Group: ANTIDIABETIC
Each Tablet contains: Glimepiride 2mg
Management of Diabetes Mellitus
10×10
Each vial contains:
Oxacillin Sodium USP equivalent to Oxacillin 1 g.
Each vial contains
Cefotaxime sodium USP equivalent to Cefotaxime 250 Mg
Each vial contains : Cefepime Hydrochloride USP Equivalent to Cefepime 500 Mg Exciepients : L-arginine
Each vial contains : Cefepime Hydrochloride USP Equivalent to Cefepime 1 Gm Exciepients : L-arginine
Each vial contains: Meropenem 500 mg and 45.1 mg of Sodium
Each vial contains : Meropenem 1 Gm and 90.2 mg of Sodium
Each vial contains : Imipenem Anhydrous 500 mg (As Imipenem monohydrate USP) Cilastatin 500 mg ( As Cilastastin Sodium USP)
Each vial contains:
Ampicillin1000 mg (as Ampicilin Sodium BP) &
Sulbactam 500 mg (as Sulbactam Sodium BP)
Each vial contains:
Ampicillin 2Gmg (as Ampiciilin Sodium BP) &
Sulbactam 1Gm (as Sulbactam Sodium BP)
Each ml contains:
Furosemide BP 10 mg
Each ml contains: DICYCLOMINE HCL 10MG / ML
Each ml contains: 25mg of RANITIDINE HCL BP equivalent TO Ranitidine inj
Each ml contains: Ondansetron HCL USP equivalent to ONDANSETRON 2MG/ML
Each ml contains: Ondansetron HCL USP equivalent to ONDANSETRON 2MG/ML
Each ml contain Metoclopramide Hydroclooride Anhydrous 5mg (As Metoclopramide Hydrochloride BP)
Therapeutic Group: ANALGESIC / ANTIINFLAMMATORY
Each Tablet contains: Diclofenac Sodium IP 50 mg
Used for management of Pain
10×10
Therapeutic Group: ANALGESIC / ANTIINFLAMMATORY
Each ml contains: Diclofenac Sodium IP 25 mg
Used for management of Pain
3ml Amp.
Therapeutic Group: ANTISPASMODICS
Each Tablet contains: Dicyclomine HCL 20 mg+ Paracetamol 500 mg
Used for management of Pain
10×10
Therapeutic Group: ANTIEMETIC / ANTIPYRETIC
Each Tablet contains: Domperidone IP 10 mg
Treatement of Gastro Intestinal Disorders
10×10
Therapeutic Group: ANTIEMETIC / ANTIPYRETIC
Each 5ml contains : Domperidone suspension 5 mg
Treatement of Gastro Intestinal Disorders
30 ml Bottle
Therapeutic Group: ANTIBIOTIC
Each Capsule contains: Doxycyclin IP 100 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each vial contains: Anhydrous Ceftazidime IP 500 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each vial contains: Anhydrous Ceftazidime IP 1 gm
Treatment of Bacterial Infections
Vial
Each vial contains:
Clavulanic Acid 100mg
(as Sterile Clavulanate PotassiumUSP)
Amoxicillin 500mg
(As Sterile Amoxicillin Sodijm BP)
Each vial contains:
Clavulanic Acid 200mg
(as Sterile Clavulanate PotassiumUSP)
Amoxicillin 1000mg
(As Sterile Amoxicillin Sodijm BP)
Vial
Each vial contains :
Piperacillin 4 gm + Tazobactum 0.5 gm IV Injection
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Ceftriaxone 1000 mg + Sulbactum 500 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Vial contains: Ceftriaxone 1g
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each vial contains:
Ceftriaxone sodium USP
equivalent to Ceftriaxone 250 mg
Therapeutic Group: ANTIBIOTIC
Each vial contains: Ceftriaxone 500 mg
Treatment of Bacterial Infections
Vial
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Cefuroxime Axetil 250mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Cefuroxime Axetil 500mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Capsule contains: Cephalexin IP 250 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Capsule contains: Cephalexin IP 500 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIALLERGIC
Each Tablet contains: Cetrizine Hydrochloride 10 mg
Treatment of Cold & Cough
10×10
Therapeutic Group: ANTIALLERGIC
Each 5ml contains: Cetrizine 5 mg
Treatment of Cold & Cough
60 ml Bottle

Therapeutic Group: ANTIARTHRITIC / ANTIINFLAMMATORY / ANALGESIC
Numol MR 4 – ACECLOFENAC 100mg + THIOCOLCHICOSIDE 4mg TABLETS
Degenerative vertebral disorders
Torticollis (Wry neck)
Dorsal pain and low back pain
Neurological disorders
NUMOL – MR 4mg Tablets – Strips of 10×10’s in blister packs
Numol MR 4 – twice a day for 5 to 7 days
Therapeutic Group: ANTIMALARIAL
Each tablet contains: Chloroquine Phosphate IP 250 mg
Treatement of Malaria
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Ciprofloxacin IP 250 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Ciprofloxacin IP 250 mg+ Tinidazole 300 mg00 mg
Treatment of Bacterial Infections
10×10

Therapeutic Group: COUGH & COLD PREPARATION
Each 5ml of REM-CC XP contains:
Ambroxol Hydrochloride 15 mg
Terbutaline Sulphate 1.25 mg
Guiaphenesin 50 mg
Menthol 2.5 mg
• Acute & Chronic asthma.
• General & Smoker’s cough.
• As an adjuvant in : Upper & lower RTI.Sinusitis & congestive condition.
Children: 2.5 ml – 5 ml 3 – 4 times daily.
Adults: 5 ml – 10 ml 3 – 4 times daily.
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Ciprofloxacin IP 500 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Ciprofloxacin IP 500 mg+ Tinidazole 600 mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Sulphamethoxazole 800mg+ Trimethoprim 160mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each Tablet contains: Sulphamethoxazole 400mg+ Trimethoprim 80mg
Treatment of Bacterial Infections
10×10
Therapeutic Group: ANTIBIOTIC
Each 5 ml contains:
Trimethoprim IP 40 mg & Sulphamethoxazole IP 200 mg
Treatment of Bacterial Infections
60 ml Bottle
Therapeutic Group: ANTIBIOTIC
EACH 5 ML CONTAINS DIPHENHYDRAMINE HYDROCHLORIDE IP 14 MG, AMMONIUM CHLORIDE IP 135 MG, SODIUM CITRATE IP 57 MG, MENTHOL IP 0.9 MG
Treatment of Cold & Cough
110 ml Bottle
Therapeutic Group: ANTIPEPTIC ULCERANT
Each capsule of Toprazol D contains : Pantoprazole (EC) 40 mg + Domperidone (SR) 30 mg
Gastric and duodenal ulcers
Gastro Esophageal Reflux Disease (GERD)
Non ulcer dypepsia
Toprazol D capsules are available in blister pack of 10 x 10’s.
It is given orally in a dose of one capsule once daily about half an hour before breakfast.
Therapeutic Group: ANTIBIOTIC
Each vial contains :
Clavulanate Potassium 200 mg
Amoxycillin Sodium 1000 mg
Recommended in infections like :
• URT / LRT
• Genito-rinery
• Bone and soft tissue
• Pre and Post operative infections.
Presented as single unit vial pack in a carton
It is recommended only for IV injection only.
Adults – Usually 1.2 g every eight hours
Children – Usually 30 mg / kg every eight hours
Infants – Usually 30 mg / kg every twelve hours
Each vial contains
Ceftriaxone sodium USP equivalent to Ceftriaxone 1 G and Sulbactam 500 mg (as Sulbactam Sodium USP)
Each vial contains
Ceftriaxone sodium USP equivalent to Ceftriaxone 1 G and Tazobactum 125 mg (as Tazobactum Sodium )
Each vial contains
Hydrocortisone 250 mg (as Hydrocortisone sodium succinate USP in a suitable buffer)
Each vial contains
Cefaperazone 250 mg(as Cefaperazone Sodium USP)
Each vial contains:
Oxacillin Sodium USP equivalent to Oxacillin 500 mg